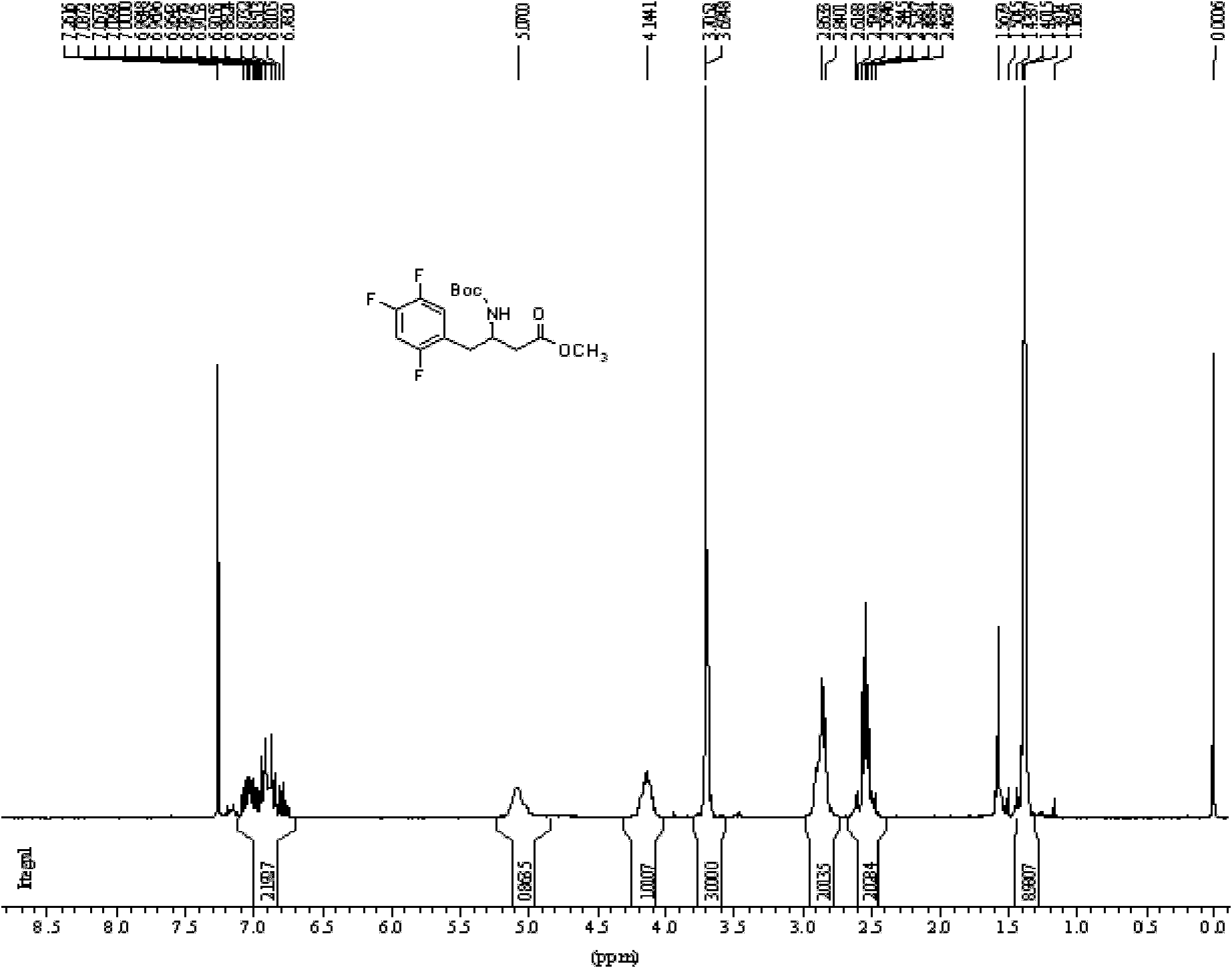
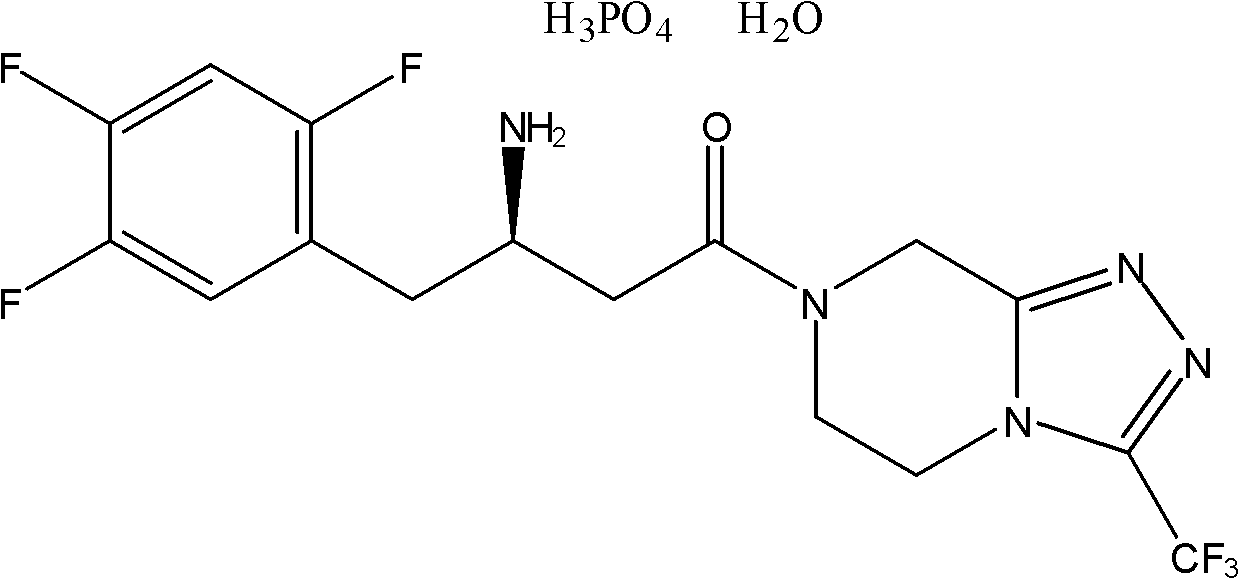
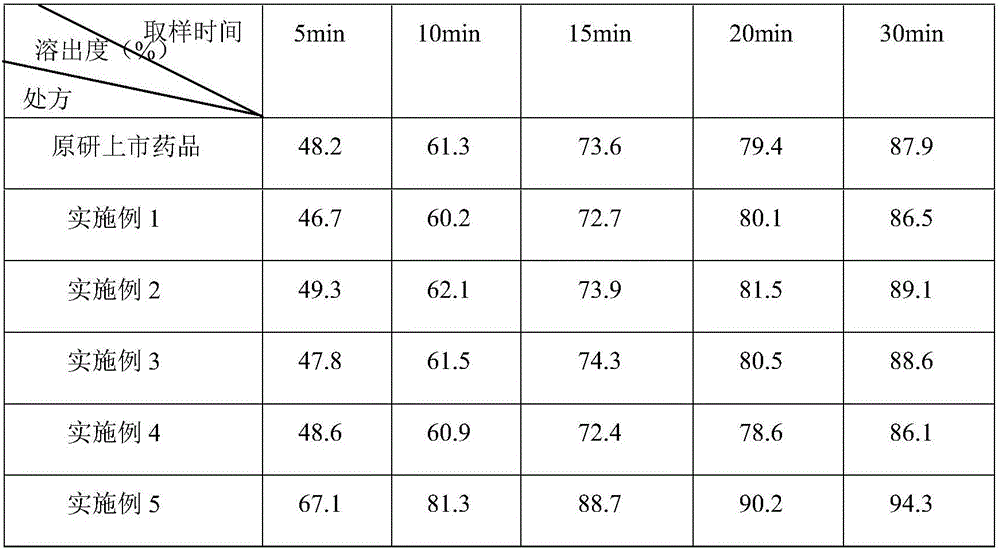
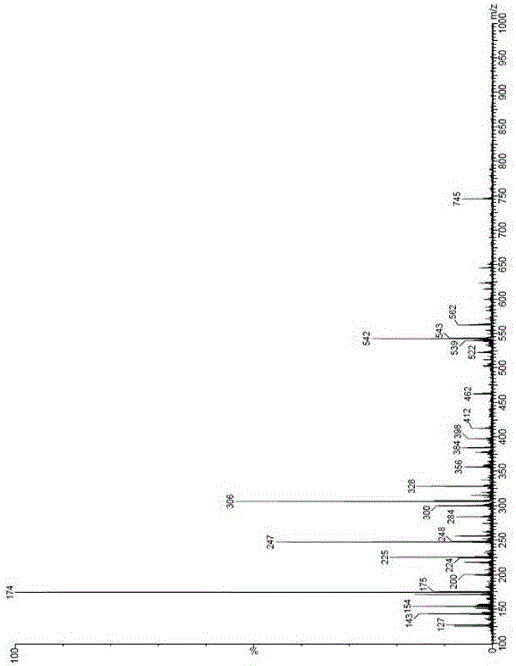
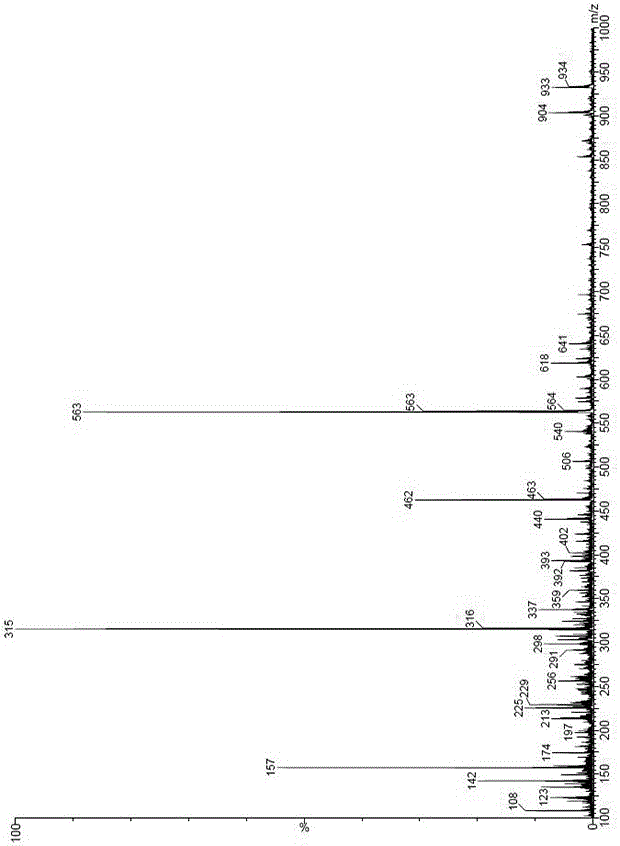
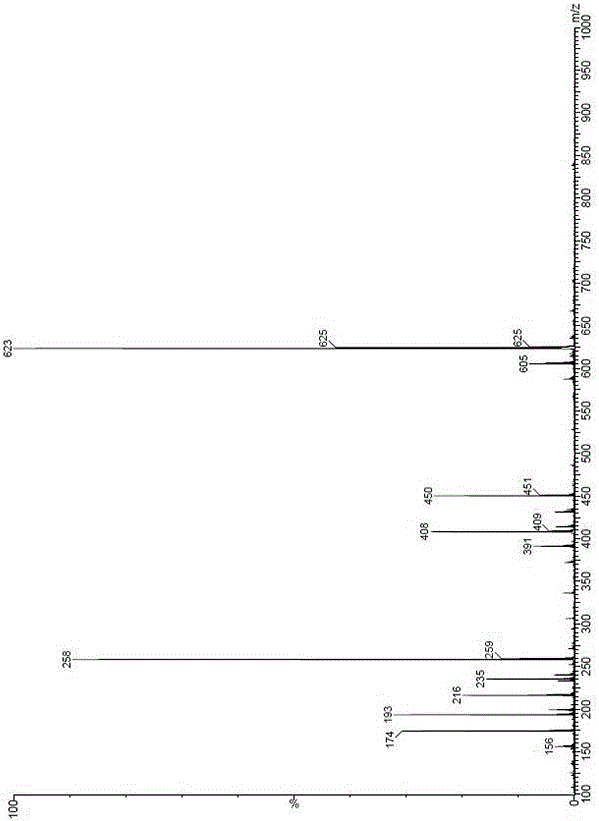
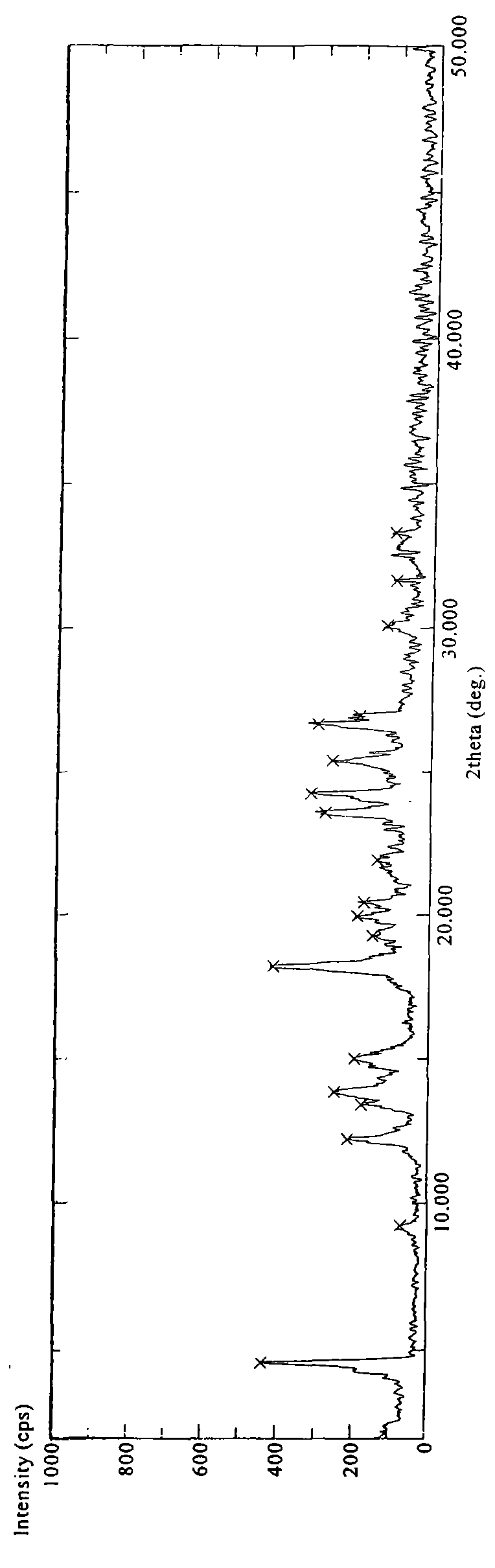
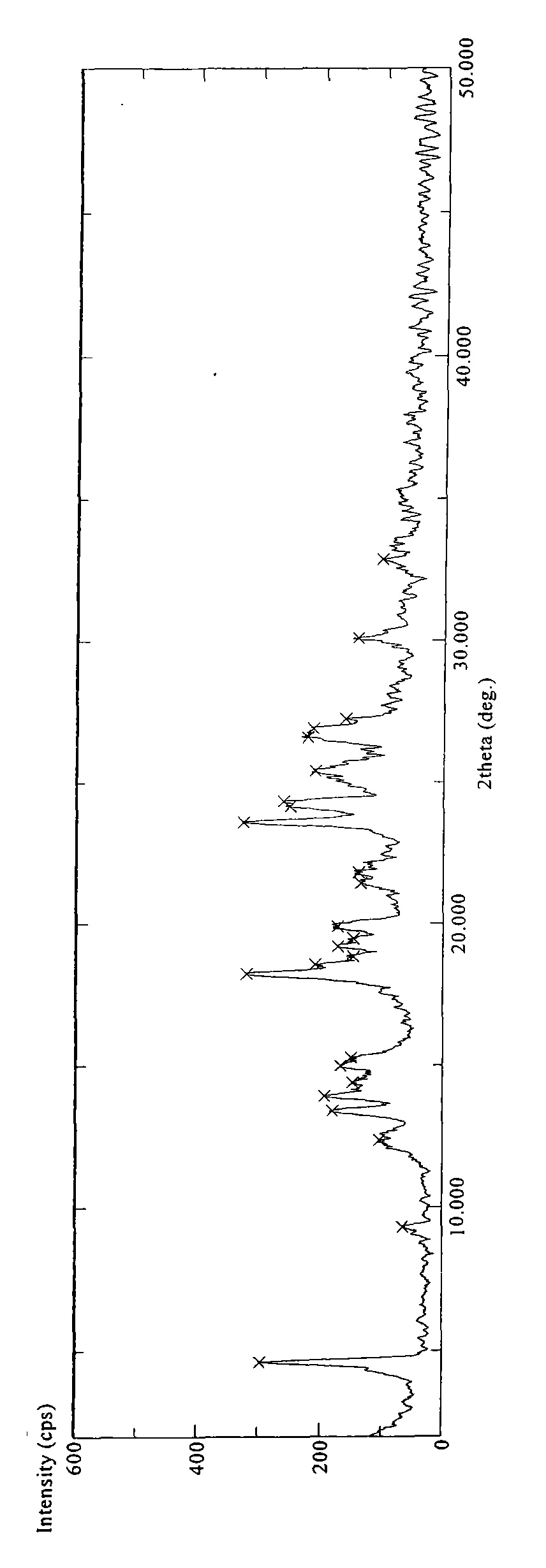
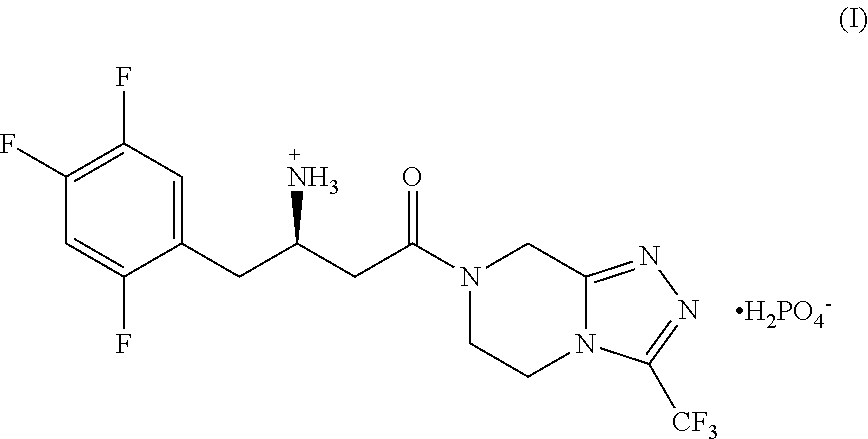
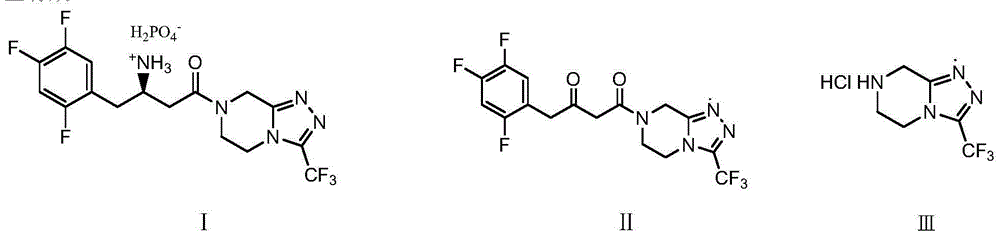
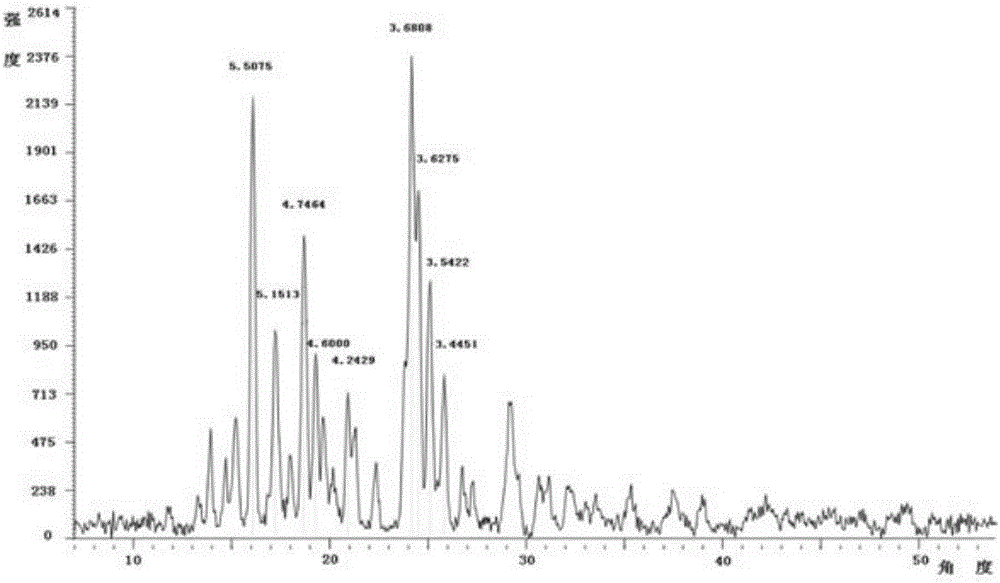
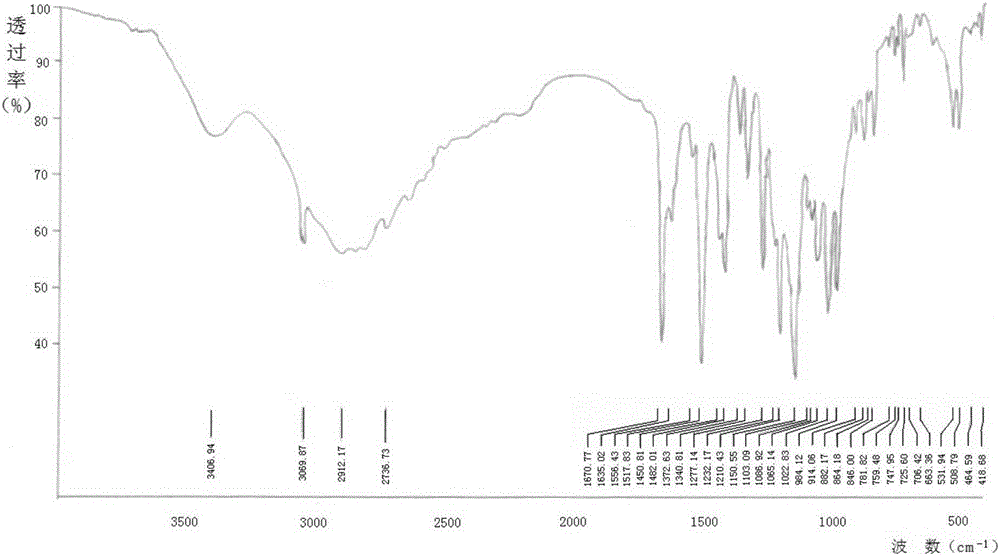
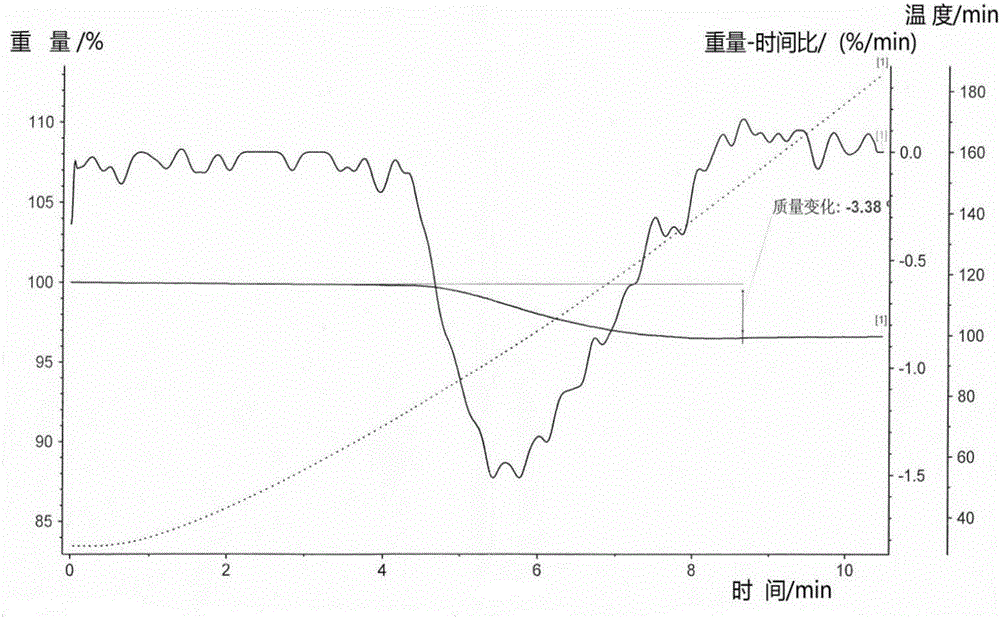
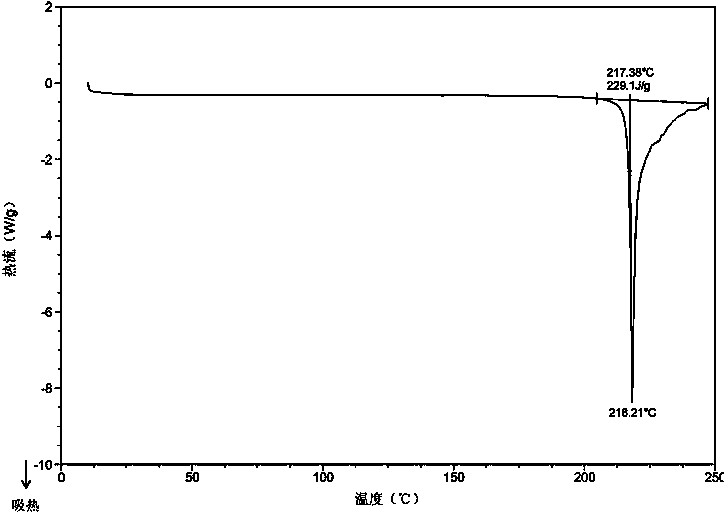
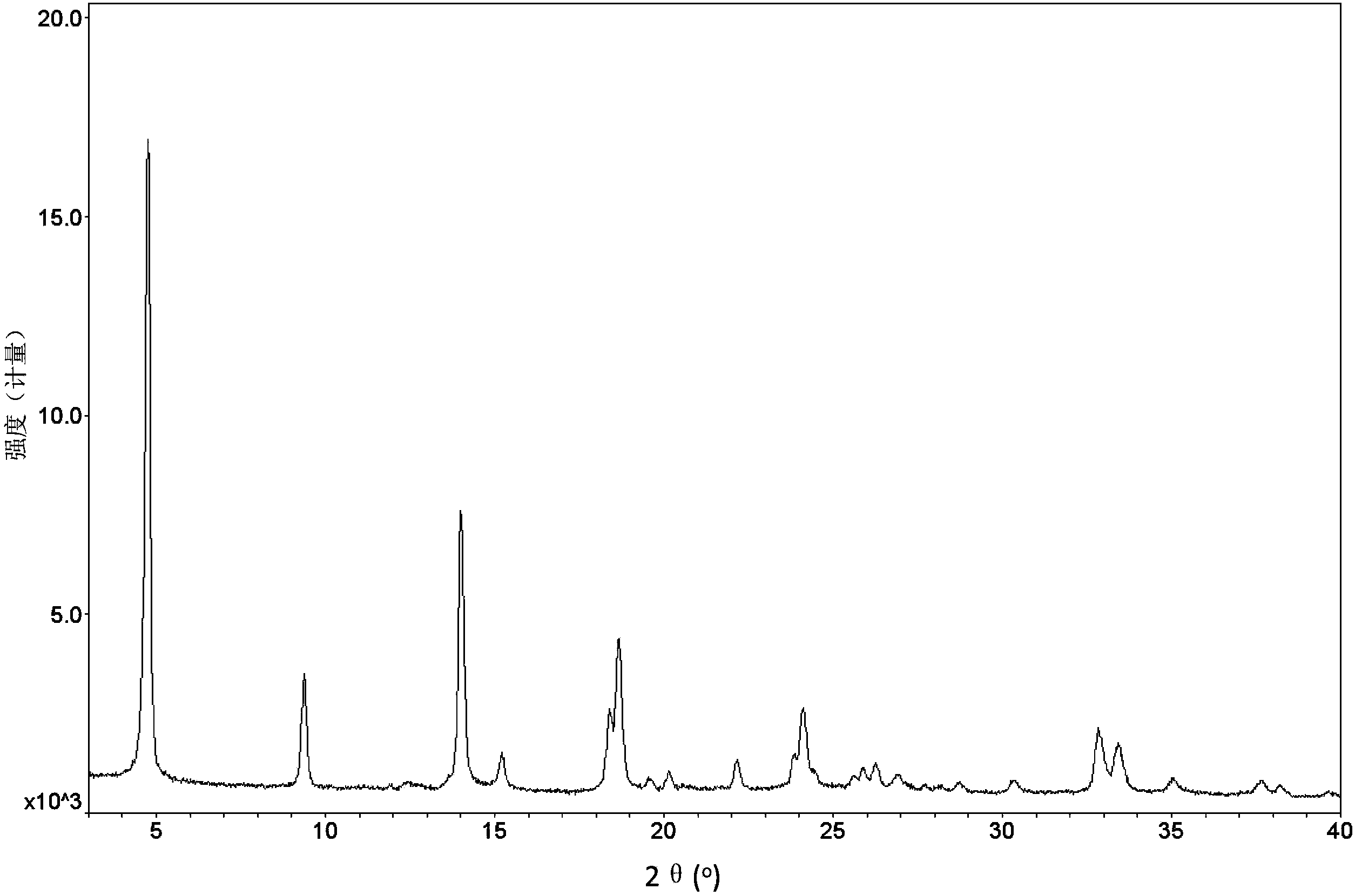
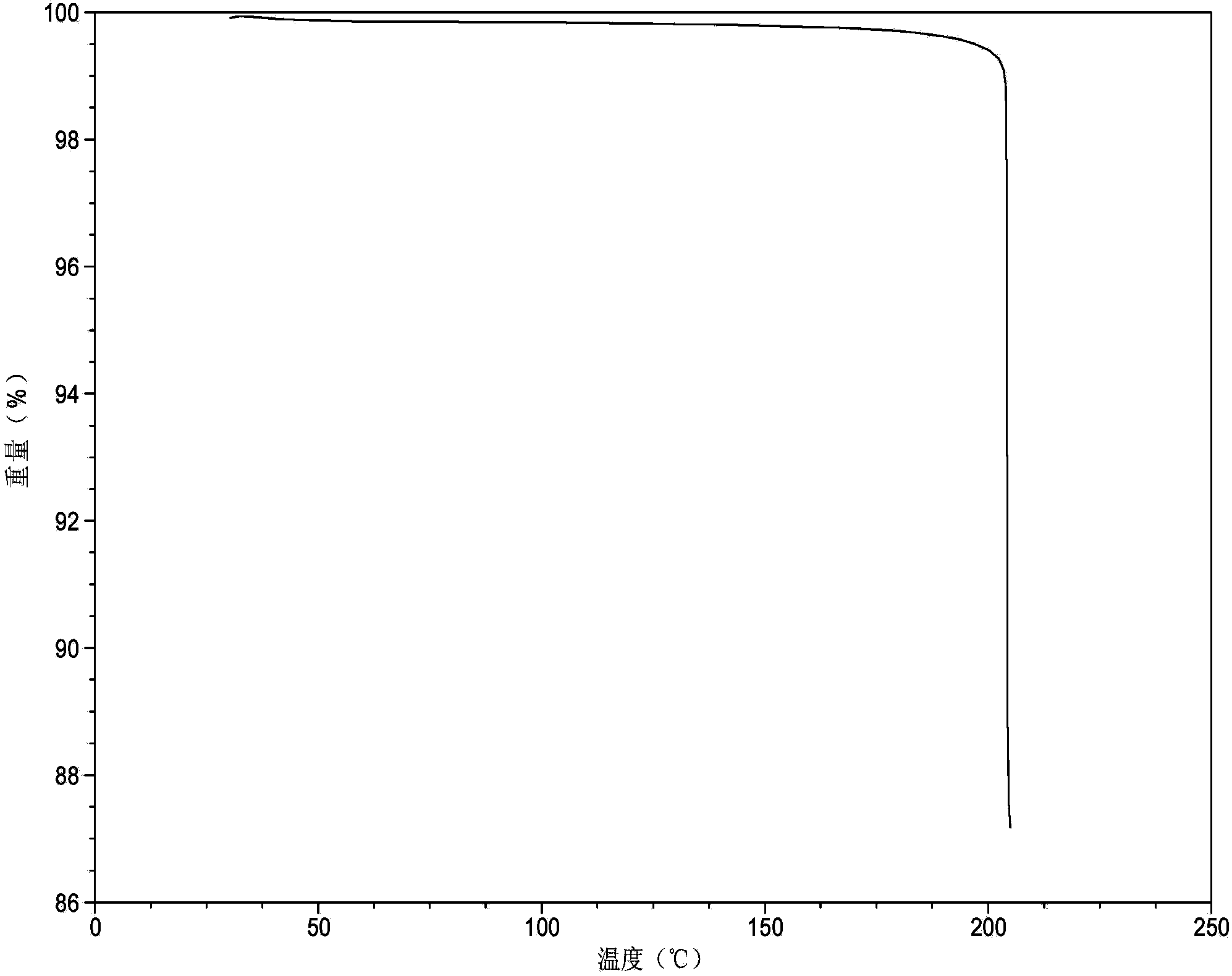
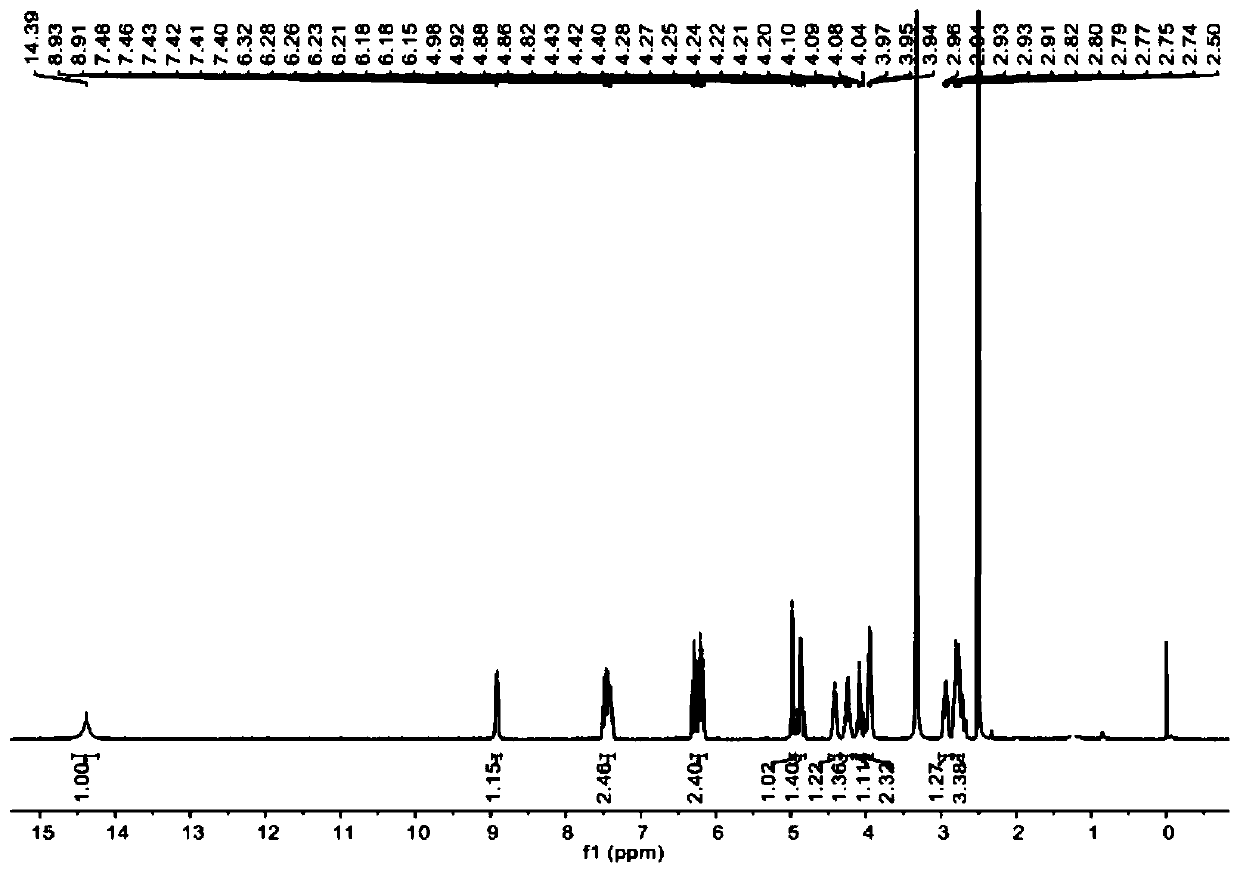
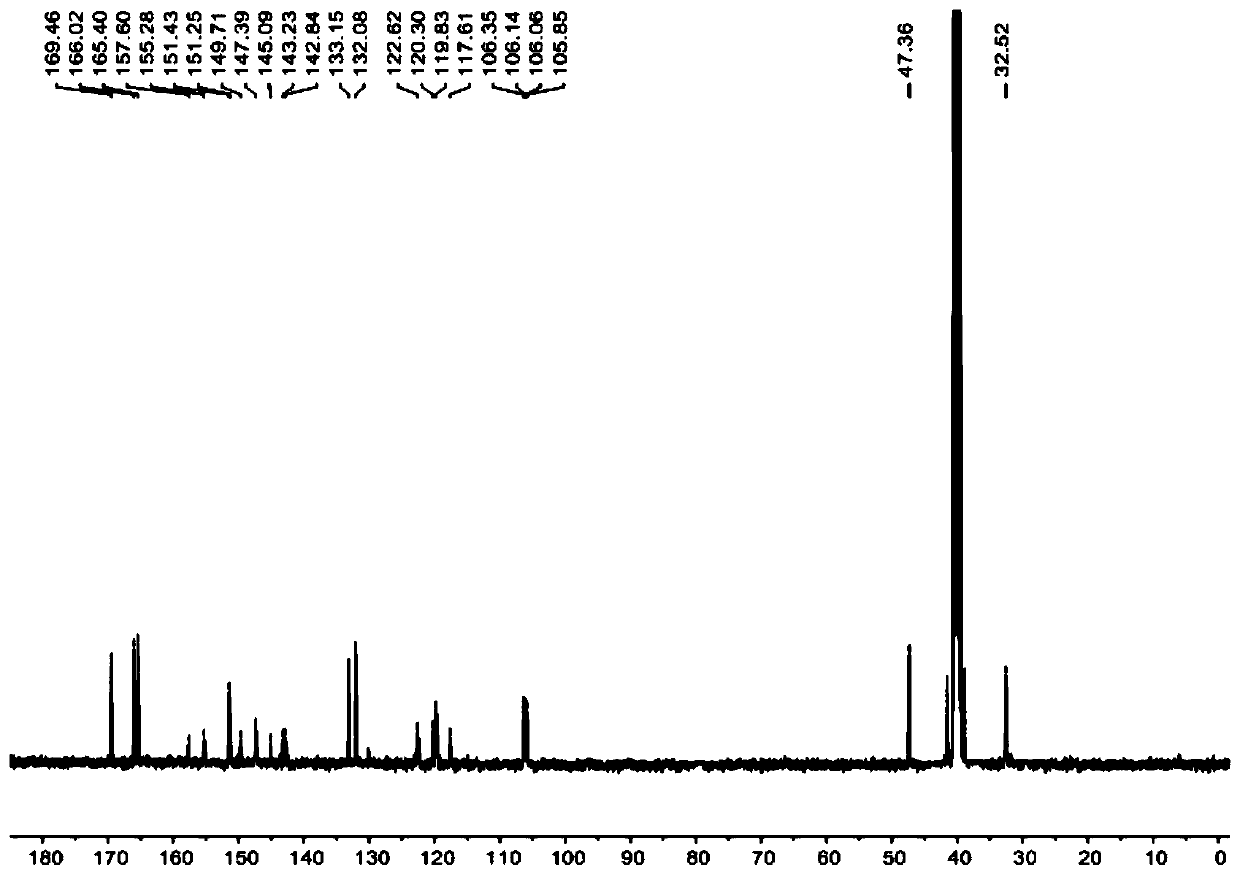
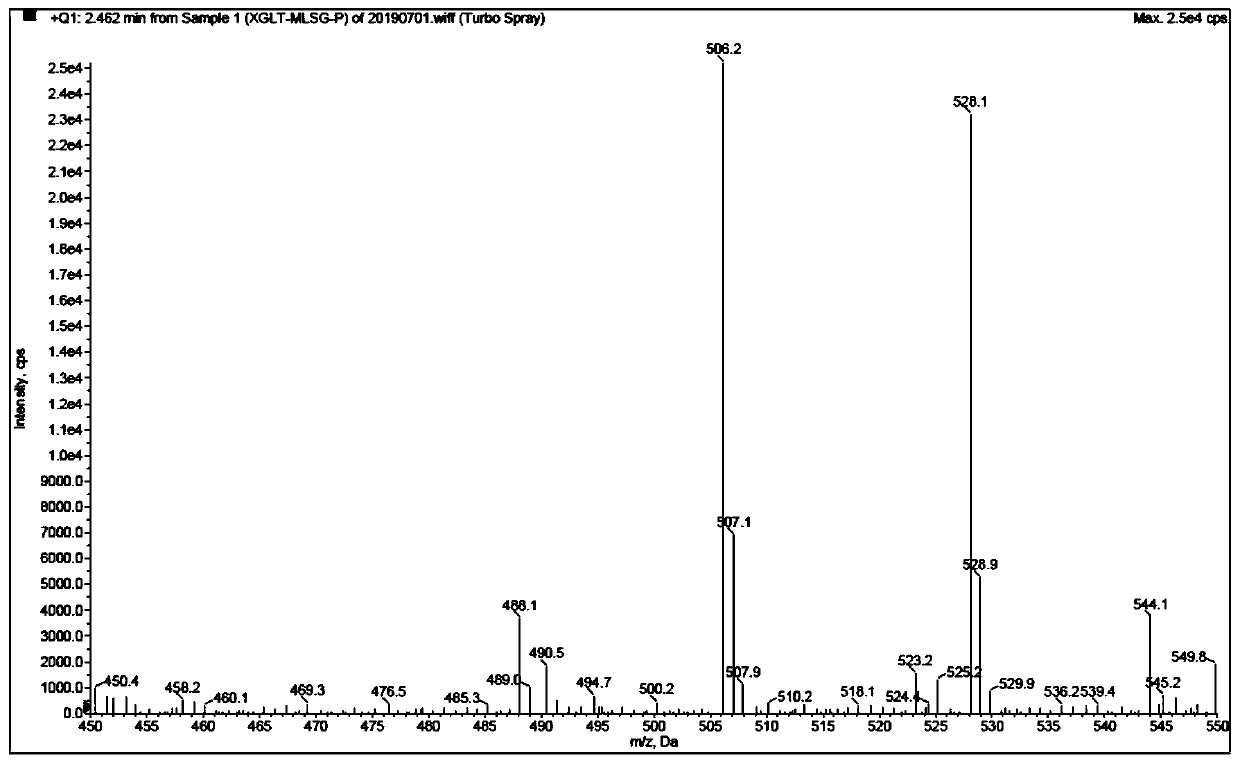
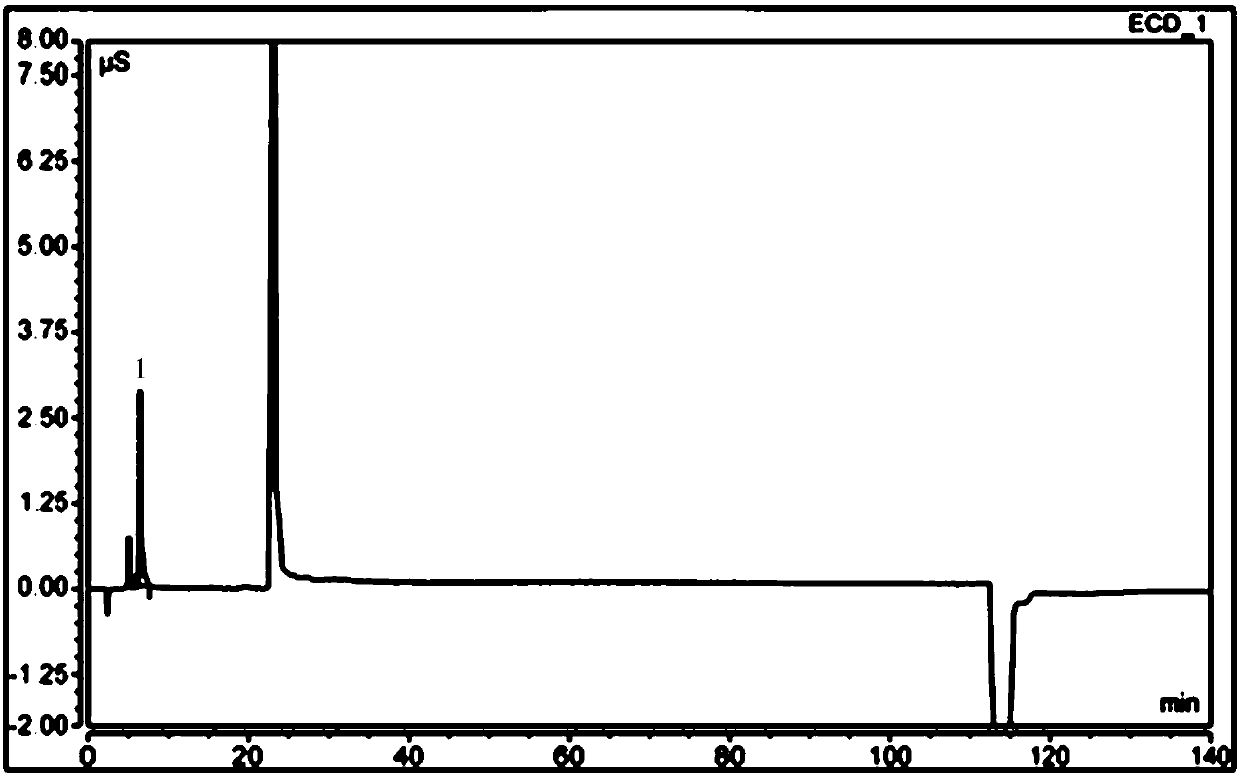
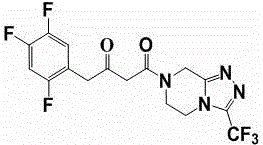
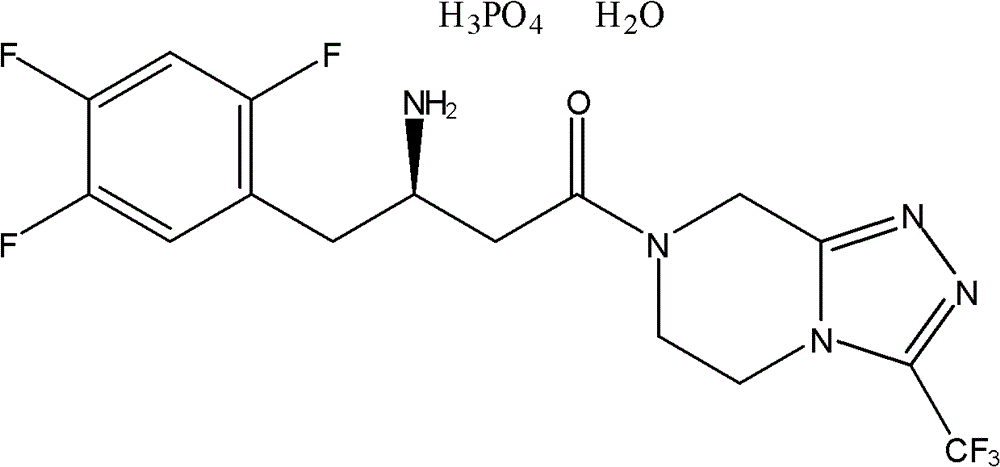
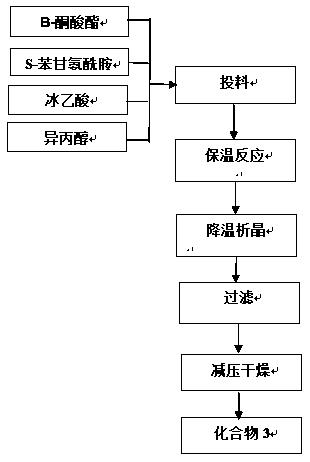
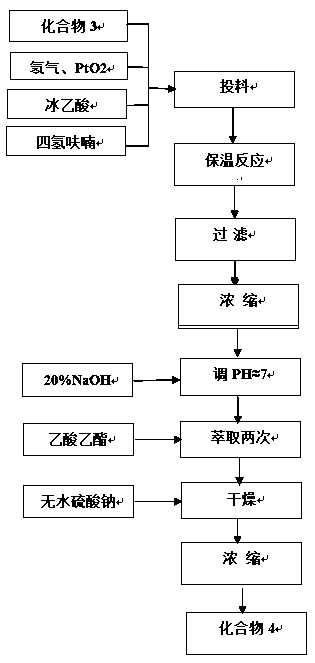
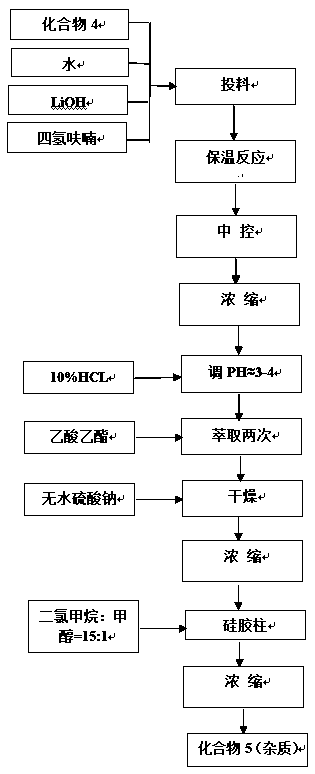
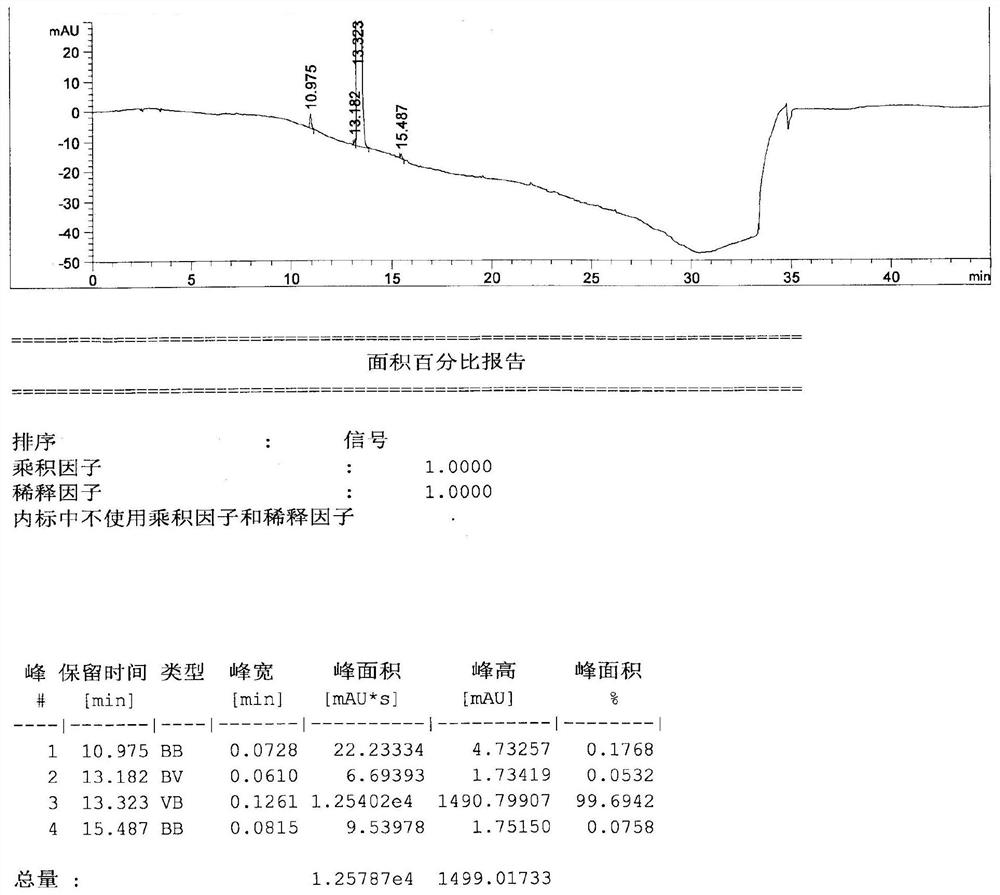
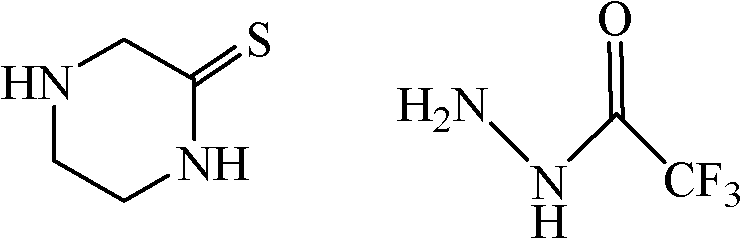Patents
Literature
57 results about "Sitagliptin Phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
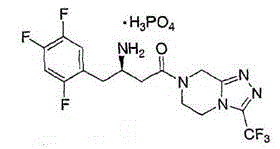
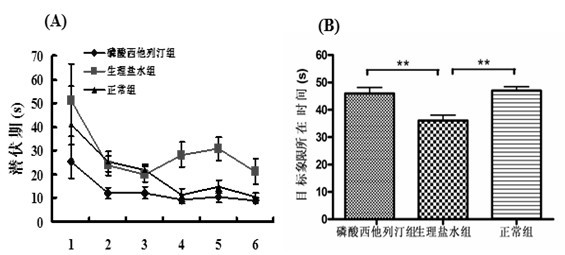
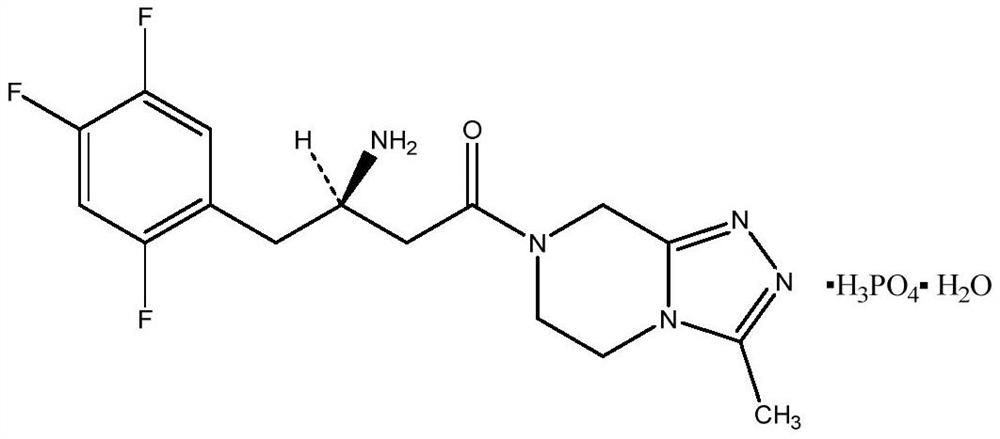
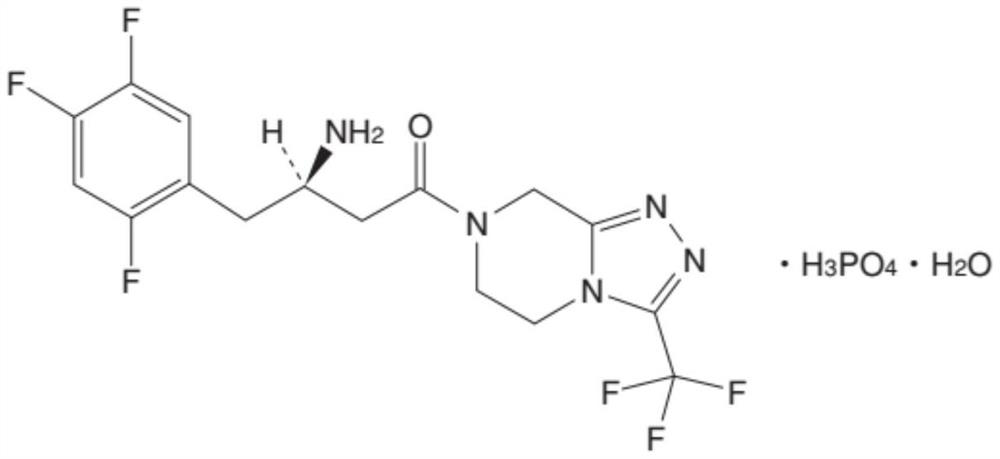
A pyrazine-derived DIPEPTIDYL-PEPTIDASE IV INHIBITOR and HYPOGLYCEMIC AGENT that increases the levels of the INCRETIN hormones GLUCAGON-LIKE PEPTIDE-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). It is used in the treatment of TYPE 2 DIABETES.
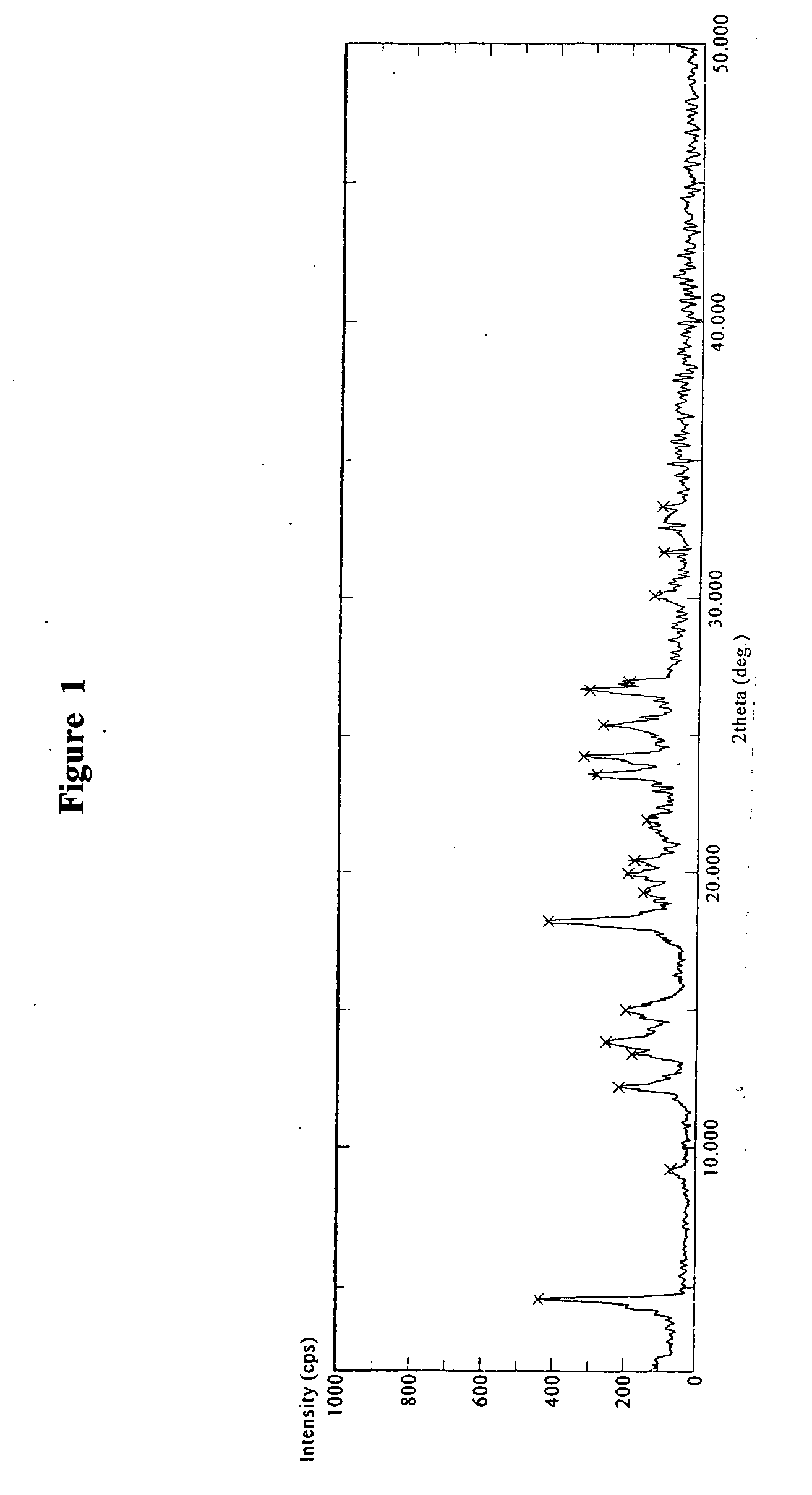
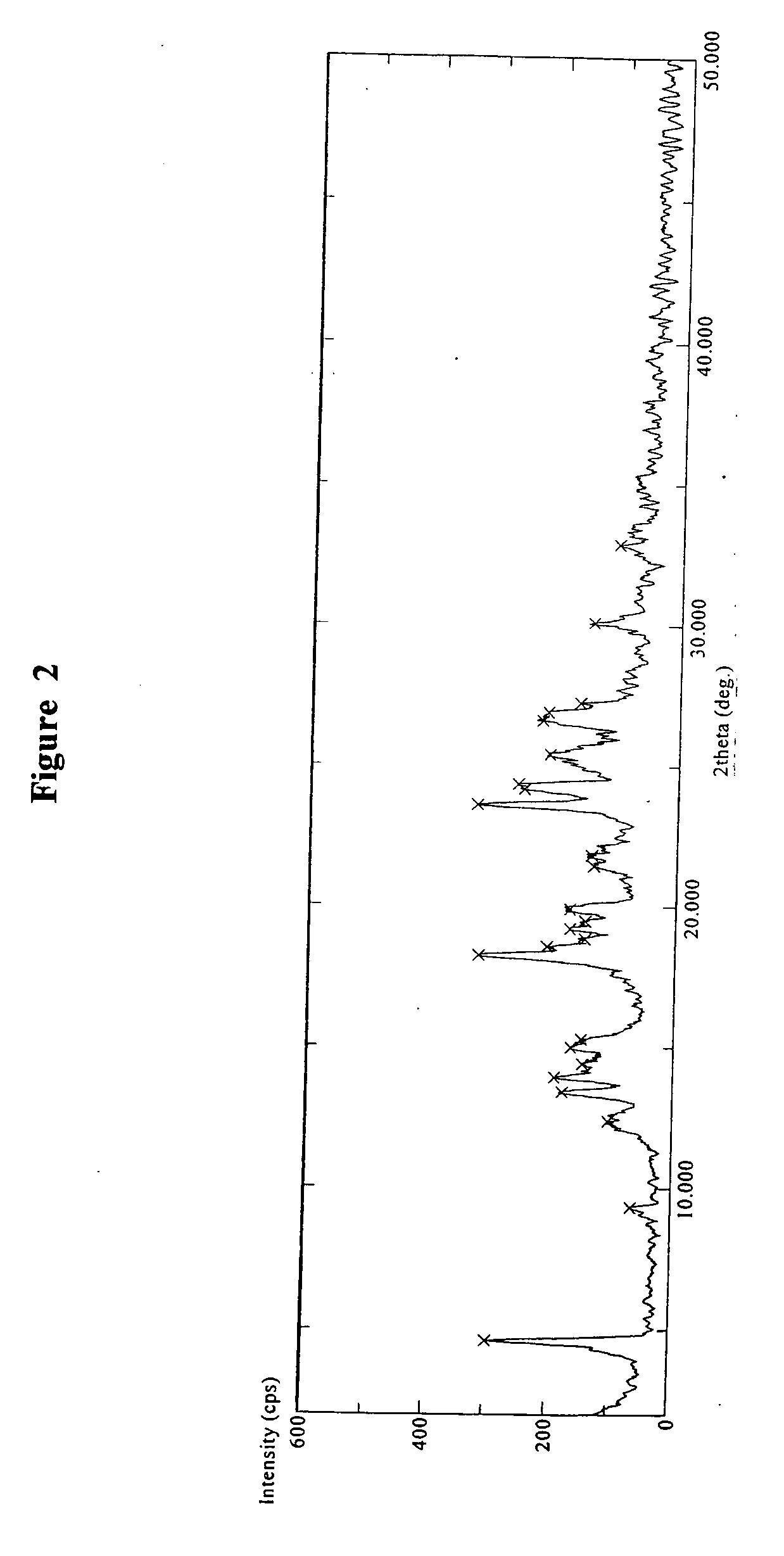
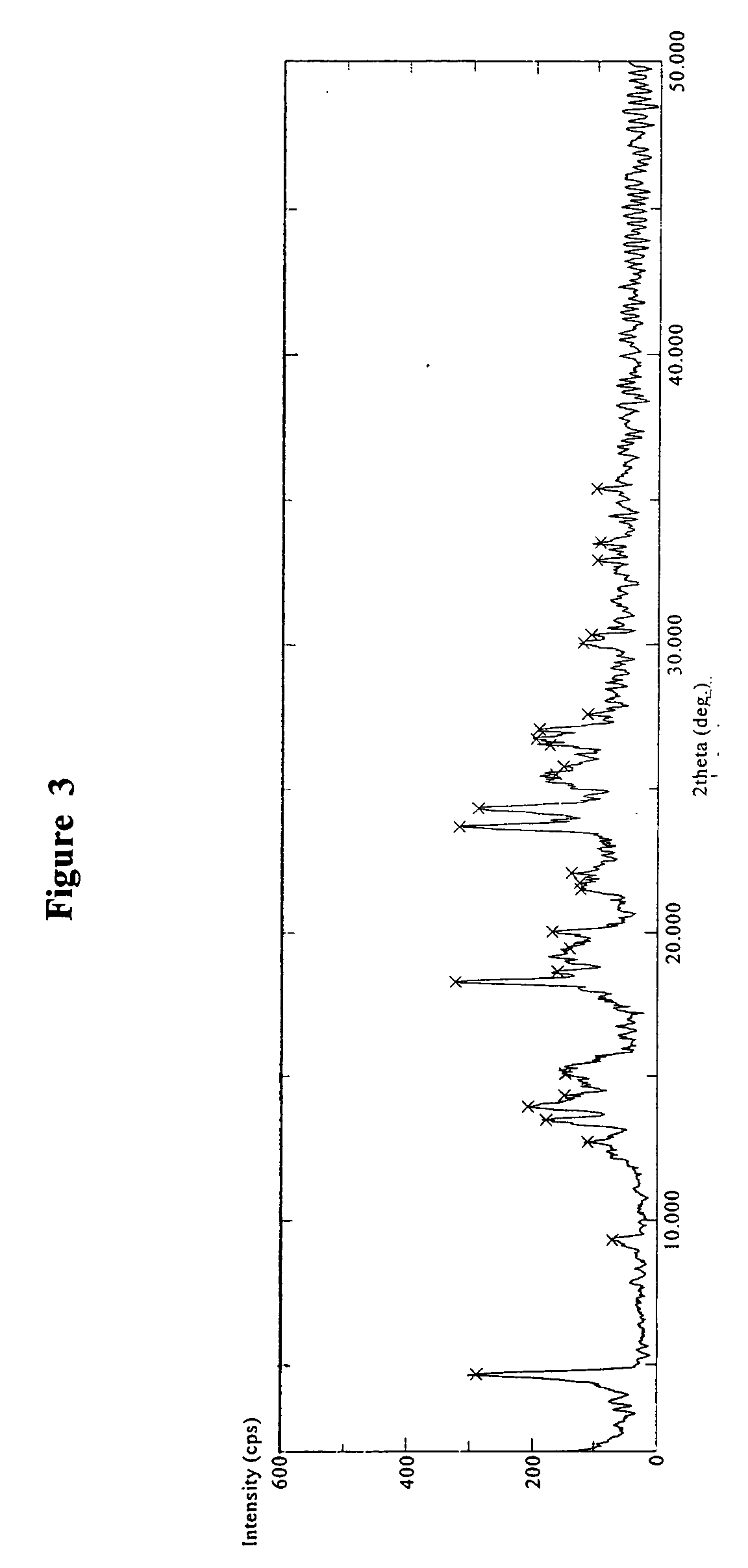
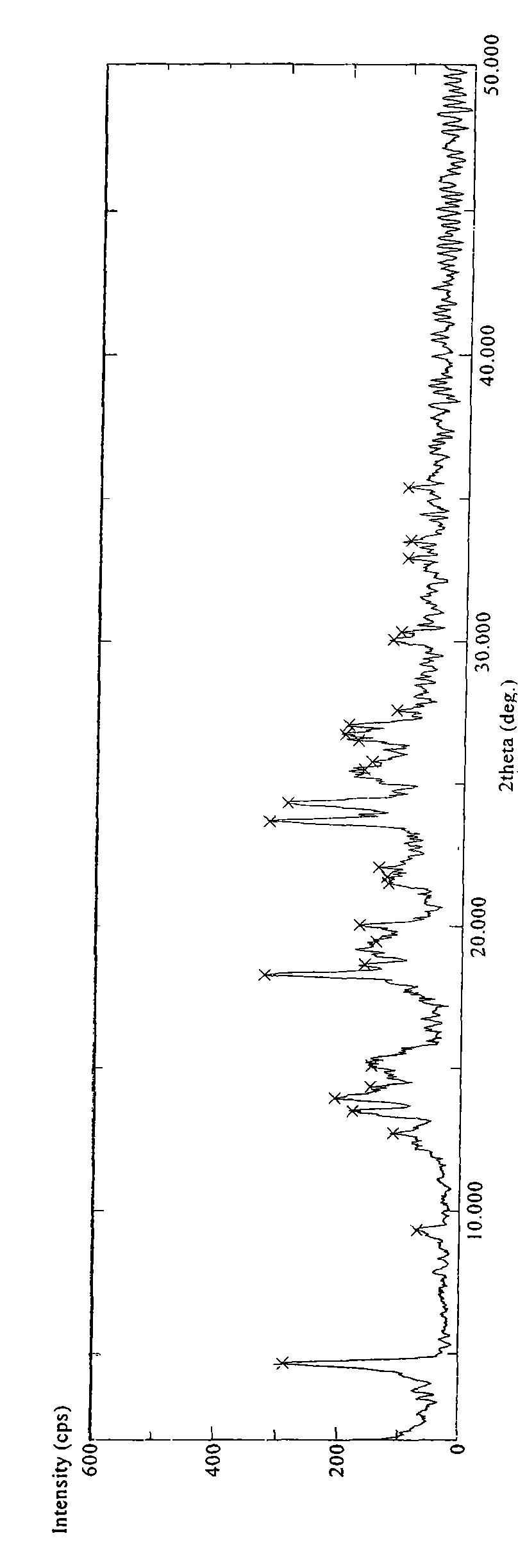
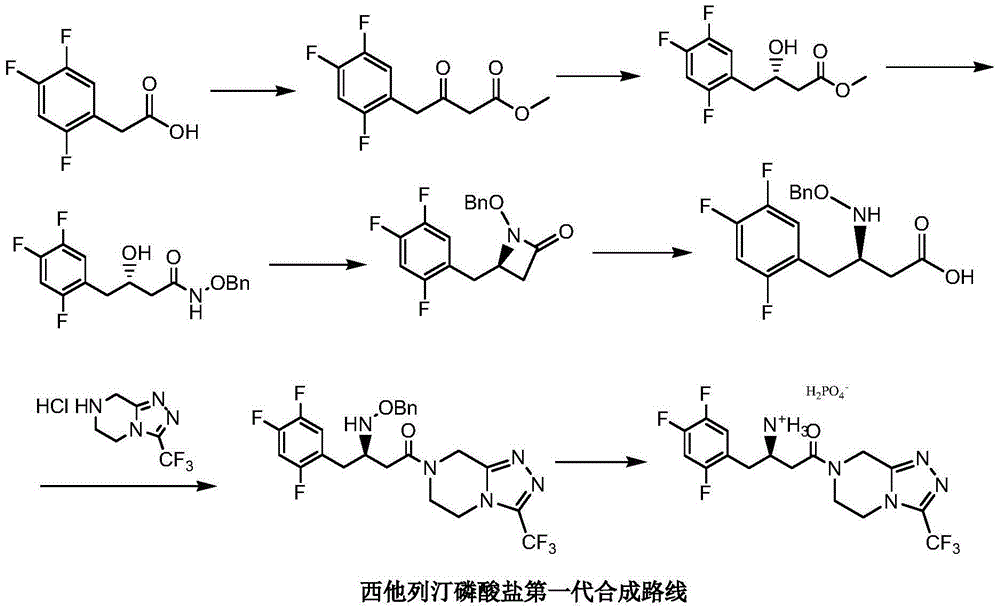
Crystalline polymorph of sitagliptin phosphate and its preparation
InactiveUS20090247532A1Big advantageBulk handlingBiocideOrganic chemistryOrganic solventDistillation
The present invention is directed to a novel polymorph form of crystalline sitagliptin phosphate, named as Form V herein. The present invention further provides processes for preparations of Form V, pharmaceutical composition comprising Form V and its use in therapy. Form V can be prepared from recrystallizing sitagliptin phosphate in a mixture of methanol and water, a mixture of acetone and water, or from distillation of a mixture of organic solvents and water followed by recrystallization in the remaining aqueous solution.
Owner:MAI DE
Novel method for synthesizing sitagliptin phosphate and derivative thereof
InactiveCN102153559ARaw materials are easy to getReduce manufacturing costOrganic chemistryBulk chemical productionChemical reactionL-Aspartate
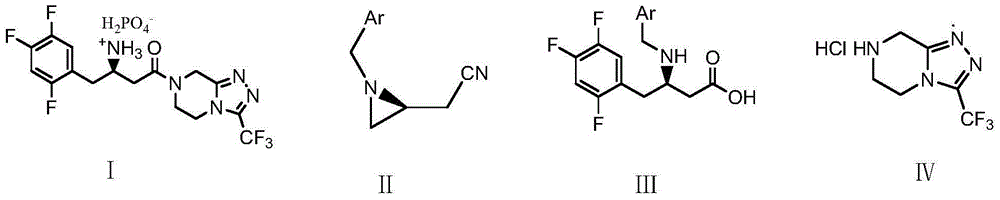
The invention discloses a novel method for synthesizing sitagliptin phosphate and a derivative thereof. The method has the advantages of readily-available raw materials, mild reaction condition, no need of using very expensive catalyst, easy control over optical purity and the like. In the novel method for synthesizing sitagliptin phosphate and the derivative thereof, L-aspartic acid is taken as a raw material, and the chemical reaction steps of amino protection, esterification, reduction, iodination or bromination, coupling, hydrolysis, acylation ammoniation, removal of protecting groups andsalt formation are performed to obtain a sitagliptin phosphate chiral salt and a hydrate thereof. The method comprises the following specific steps of: performing amino protection, esterification, reduction and iodination on L-aspartic acid serving as the raw material to obtain a compound A shown as a formula I; undergoing a coupling reaction on the compound A and a compound B shown as a formula II to obtain a compound C shown as a formula III; and performing hydrolysis, acylation ammoniation, removal of protecting groups and a salt-forming reaction on the compound C to obtain a sitagliptin phosphate hydrate.
Owner:NANJING UNIV OF TECH
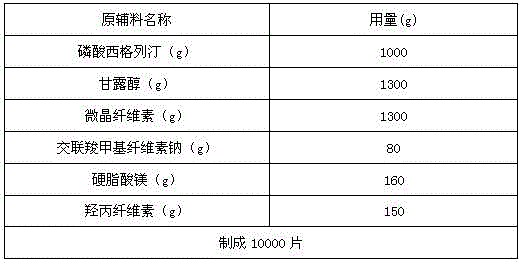
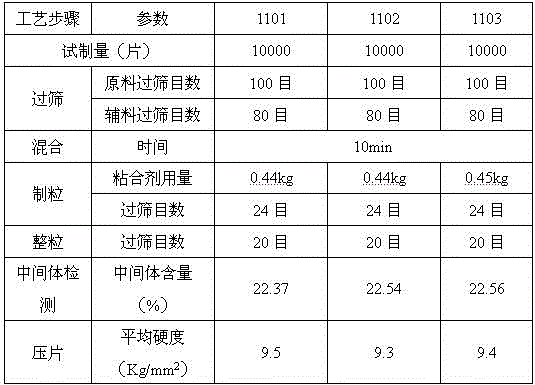
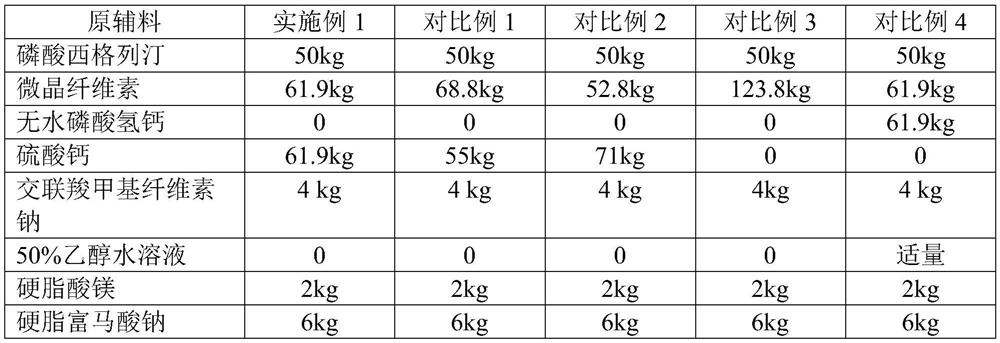
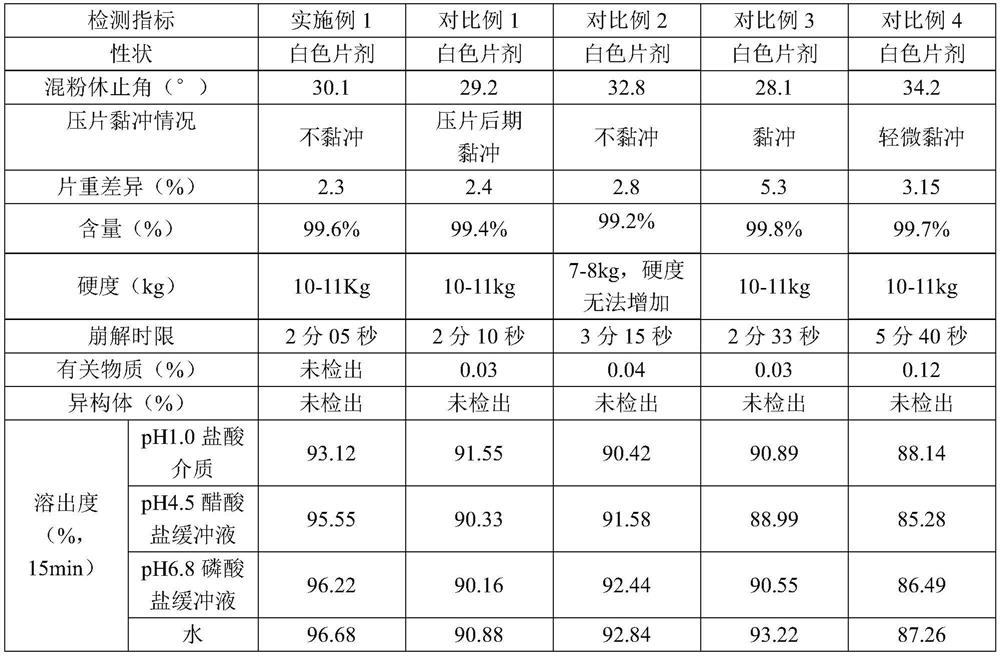
Sitagliptin phosphate tablet and preparation method thereof
InactiveCN106822017ASynthetic raw materials are readily availableFast disintegrationOrganic active ingredientsMetabolism disorderAdhesiveFiller Excipient
The invention belongs to the technical field of drug preparation and specifically relates to a sitagliptin phosphate tablet and a preparation method thereof. The sitagliptin phosphate tablet comprises the following raw materials in parts by weight: 30 parts of sitagliptin phosphate, 55-70 parts of filler, 0-5 parts of adhesive, 1.5-3.0 parts of disintegrating agent and 1.5-5.0 parts of lubricating agent; the filler is selected from one or more of microcrystalline cellulose, sugar and inorganic salt; the adhesive is selected from one or more of hydroxypropyl methylcellulose, hydroxypropylcellulose, povidone K30 and starch; the disintegrating agent is selected from one or more of crosslinking sodium carboxymethylcellulose, sodium carboxymethyl starch and polyvinylpolypyrrolidone. Compared with the prior art, the sitagliptin phosphate tablet provided by the invention has the technical effects of easily acquired compound raw materials, high disintegration speed, high dissolution rate, high stability, simple steps of preparation method, easily controlled technological process, short production period, low production cost and suitability for industrialized application.
Owner:CISEN PHARMA
Sitagliptin phosphate impurities, method for preparing same and application of sitagliptin phosphate impurities
ActiveCN106349245ASimple and fast operationModerate reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationPhosphoric acidSitagliptin Phosphate
The invention discloses sitagliptin phosphate impurities, a method for preparing the same and application of the sitagliptin phosphate impurities. The sitagliptin phosphate impurities are sitagliptin phosphate impurities A, sitagliptin phosphate impurities B and sitagliptin phosphate impurities C. The relevant sitagliptin phosphate impurities, the method and the application have the advantage of important monitoring significance on industrial production of sitagliptin phosphate crude medicines.
Owner:CHONGQING ZEN PHARMACEUTICAL CO LTD
Sitagliptin phosphate composition tablet and preparation method thereof
InactiveCN104644578ASimple stepsThe process is easy to controlOrganic active ingredientsMetabolism disorderStearic acidMannitol
The invention relates to the field of pharmacy, and discloses a sitagliptin phosphate composition tablet and a preparation method thereof. The sitagliptin phosphate composition tablet disclosed by the invention comprises the following effective components: sitagliptin phosphate, mannitol, microcrystalline cellulose, croscarmellose sodium, magnesium stearate and 5% hydroxy propyl cellulose. The sitagliptin phosphate composition tablet is available in synthesis materials, low in cost, high in disintegrating speed, high in dissolution rate, good in stability, accurate in dosage, convenient to take and convenient to carry. The method for preparing the sitagliptin phosphate composition tablet is simple in steps, short in production cycle, low in production cost and suitable for industrialized application; and the technological process is easy to control.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Pharmaceutical compositions of combinations of dipeptidyl peptidase-4 inhibitors with simvastatin
InactiveUS20140093564A1BiocideMetabolism disorderDipeptidyl peptidaseDipeptidyl peptidase-4 inhibitor
The present invention is directed to novel pharmaceutical compositions comprising fixed dose combinations of a dipeptidyl peptidase-4 inhibitor (DPP-4 inhibitor), or a pharmaceutically acceptable salt thereof, and simvastatin, or pharmaceutically acceptable salt thereof, methods of preparing such pharmaceutical compositions, and methods of treating Type 2 diabetes and hypercholesterolemia with such pharmaceutical compositions. In particular, the invention is directed to pharmaceutical compositions comprising fixed-dose combinations of sitagliptin phosphate and simvastatin.
Owner:MERCK SHARP & DOHME LTD
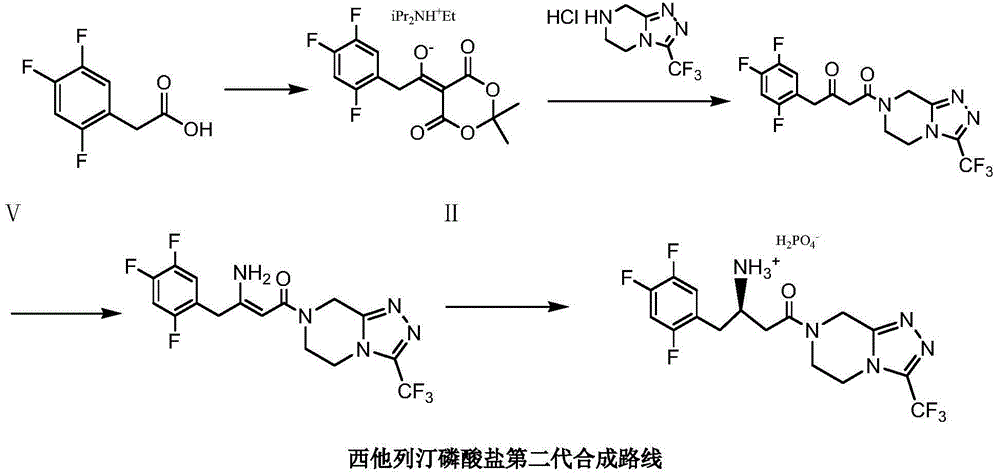
Low cost method for preparing sitagliptin phosphate salt key intermediate
ActiveCN104987338AReduce manufacturing costLess waste waterOrganic chemistryGrignard reagentPyrazine
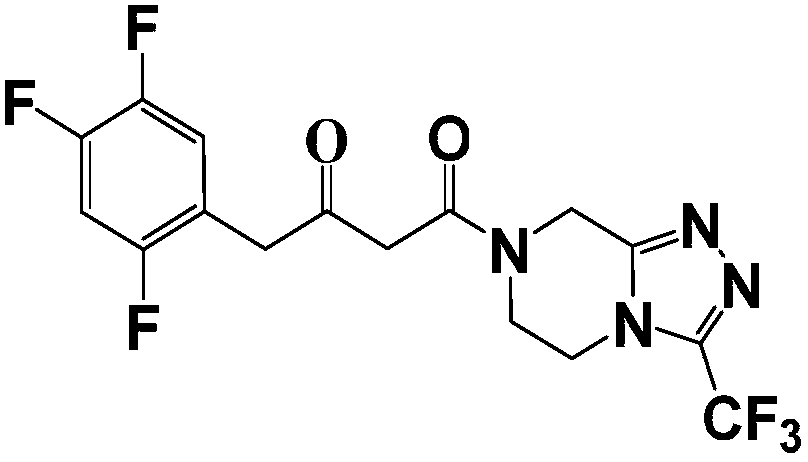
The invention relates to a low cost method for preparing a sitagliptin phosphate salt key intermediate. The method comprises the steps of: with existence of suitable solvents and inorganic bases, preparing 3-oxalysine-[3-(trifluoromethyl)-5,6-dihydrogen[1,2,4]triazolo[4,3-a]pyrazinyl-7(8H)]propionitrile(IV) by reacting cyanoethanoate ester with 5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazine hydrochloride(III)amidation and removing alcohol; and compound IV then reacting with a Grignard reagent that is prepared by 2,4,5-trifluoro-1-halogenated methyl benzene and magnesium, so as to obtain 4-oxalysine-4-[3-(trifluoromethyl)-5,6-dihydrogen[1,2,4]triazolo[4,3-a]pyrazinyl-7(8H)]-1-(2,4,5-trifluorophenyl)-2-butanone(II). According to the method provided by the invention, the expensive Michaelis acid, trimethylacetyl chloride and diisopropylethylamine are not used; the raw material used is cheap and easy to obtain and has less waste water in the production process; the product is high in yield, has few impurities, and is low in cost.
Owner:XINFA PHARMA
A kind of transaminase and its application in the synthesis of sitagliptin intermediate
ActiveCN105018440BEasy to recycleHigh optical purityBacteriaTransferasesSitagliptin PhosphateRecombinase
The invention provides a new aminotransferase, a gene of the new aminotransferase, a recombinant expression vector containing the gene, a recombinant expression transformant, a recombinase, a preparation method of the recombinase, and application of the aminotransferase to preparation of active chiral amine by performing asymmetrical transamination on a carbonyl compound. The aminotransferase is derived from mycobacterium (mycobacterium vanbaalenii) PYR-1, and is applied to preparation of (R)-3-amino-4-(2, 4, 5-trifluorophenyl)-methyl butyrate. Compared with other preparation methods, the preparation methods provided by the present invention have the advantages that catalyzed and prepared products are high in concentration, enantioselectivity is high, reaction conditions are mild, operations are simple and convenient, enlargement is easy and the like, so that the industrial application prospect in production of sitagliptin phosphate is excellent.
Owner:弈柯莱(台州)药业有限公司
Sitagliptin phosphate crystal and preparation method and application thereof
ActiveCN105175422AImprove solubilityImprove stabilityOrganic active ingredientsMetabolism disorderSolubilityState of art
The invention provides a crystal-type sitagliptin phosphate monohydrate and a preparation method, pharmaceutical composition and application thereof. The solubility of the sitagliptin phosphate crystal is high and obviously higher than that of a sitagliptin phosphate monohydrate in the prior art, and the sitagliptin phosphate crystal is good in both stability and hygroscopicity under the conditions of high temperature, high humidity and illumination. Compared with the prior art, the crystal-type sitagliptin phosphate monohydrate has the advantages that the solubility is higher, and the great significance for improving the pharmaceutical effect and reducing the pharmaceutical loading capacity is achieved; the preparation method is easy to operate, good in repeatability and beneficial for cost control in industrialized production and has the extremely high economic value; the time period of industrialized mass production is greatly shortened, the production efficiency is improved, the production energy consumption is reduced, and the cost of industrialized mass production is lowered.
Owner:SHENZHEN HAIBIN PHARMA +1
Method for preparing sitagliptin phosphate anhydrous crystal form I
ActiveCN103421011AAvoid formingSimple processOrganic active ingredientsOrganic chemistrySingle crystalCrystallization temperature
The present invention relates to a method for preparing an anhydrous crystal form I of sitagliptin phosphate. The method comprises: crystallizing by stirring a suspension of sitagliptin phosphate solid at a crystallization temperature, then separating the crystallized crystals, washing, and drying so as to obtain an anhydrous crystal form I of the sitagliptin phosphate, wherein the solvent for the suspension of the sitagliptin phosphate solid is selected from acetone or acetonitrile, or the solvent for the suspension of the sitagliptin phosphate solid is selected from a mixture of a C1-4 alkanol and water, a mixture of ethylene glycol and water, a mixture of acetone and water, or a mixture of acetonitrile and water. A single crystal form of sitagliptin phosphate in an anhydrous crystal form I can be prepared by the method of the present invention. The method facilitates the control of product quality and the establishment of a quality standard, and has advantages such as a simple and convenient crystallization process, mild reaction conditions, and a high product yield without a high temperature reaction for a long time.
Owner:ZHEJIANG HISOAR PHARMA +1
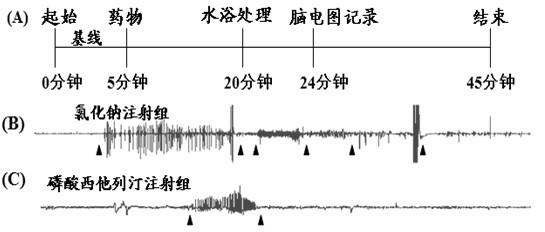
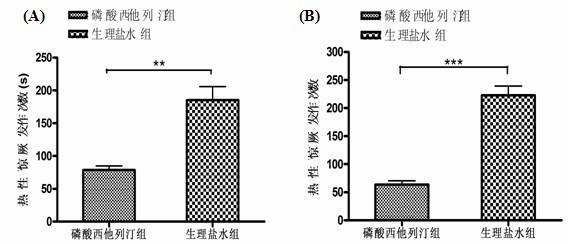
Application of sitagliptin phosphate in preparation of medicament for preventing and treating febrile convulsion
InactiveCN102600161AActive ingredients are clearLow costOrganic active ingredientsNervous disorderFebrile convulsionsSide effect
The invention belongs to technical field of medicines and relates to application of sitagliptin phosphate in preparation of a medicament for preventing and treating febrile convulsion. Currently, the sitagliptin phosphate has obvious medicament effect of clinically treating the febrile convulsion, but various serious side effects can be produced to damage children, and the tolerance and the addiction can be produced after long-time use. The product disclosed by the invention is definite in active ingredients, lower in cost and easy to implement.
Owner:WUHAN UNIV
Production method suitable for enzymatic synthesis of sitagliptin phosphate
PendingCN110791538AMeet quality requirementsSuitable for mass productionFermentationBiotechnologyEnzymatic synthesis
The invention discloses a production method suitable for enzymatic synthesis of sitagliptin phosphate. The method is characterized by comprising the following steps: (1) carrying out fermentation culture on transaminase cells with catalytic activity, performing centrifuging to obtain bacterial sludge, performing crushing twice, and directly putting crushed powder into a reaction kettle to carry out catalytic reaction with a substrate, wherein the addition amount is 45-85 wt% (based on the amount of the bacterial sludge / the substrate); (2) using partially-recovered DMSO in the catalytic reaction, wherein the total impurity content is less than or equal to 5%, the specific gravity is required to be 1.0-1.1, and the ratio of the DMSO to added fresh DMSO is 1:(0.2-0.8); (3) adding ethyl acetate for extraction after adjusting the acidity to ensure that the impurity content in mother liquor is lower than 0.1%; and (4) adding recycled isopropanol (the content is 70%-90%) into a salifying reaction. Compared with common reported enzyme catalysis methods, the process method can ensure that the finished product meets the quality requirements of raw material medicines, saves the production cost to the maximum extent, and is suitable for large-scale production and application.
Owner:HUBEI HONGYUAN PHARMA
Method for preparing sitagliptin phosphate side chain
InactiveCN101973997AShort routeRaw materials are cheap and easy to getOrganic chemistryPhosphorus pentasulfideThioketone
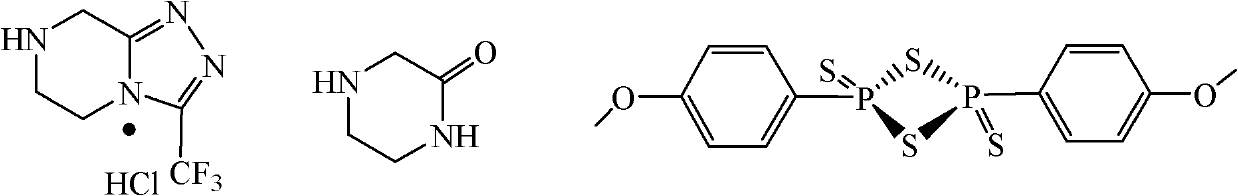
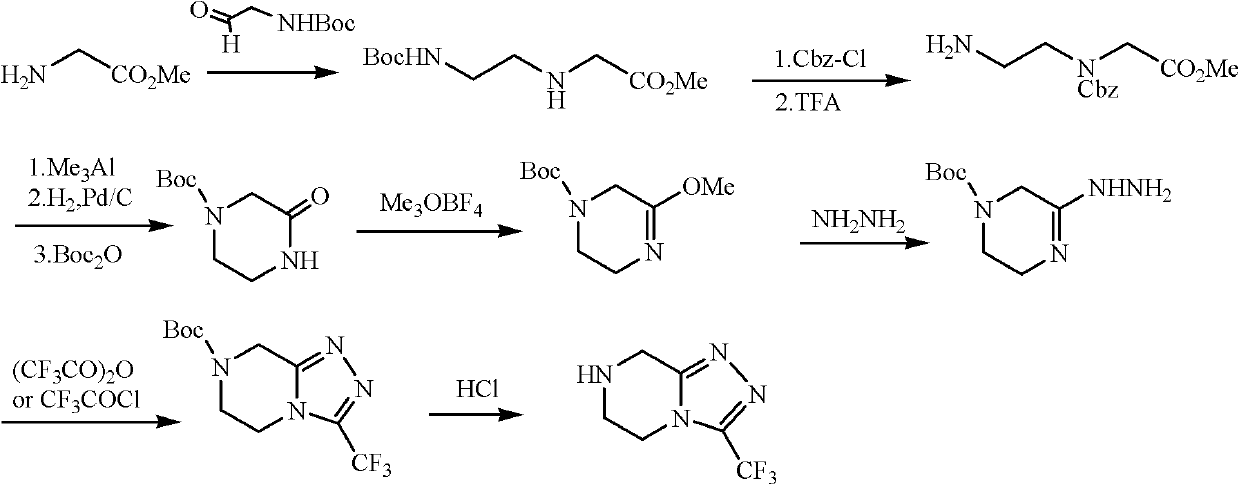
The invention discloses a method for preparing a sitagliptin phosphate side chain which has a structure shown by a formula 1. The method comprises the following steps of: a) preparing 2-piperazine thioketone with structure shown by a formula 4 by vulcanizing 2-piperazine ketone with structure shown by a formula 2 with a vulcanizing reagent, wherein the vulcanizing reagent is phosphorus pentasulfide or a Lawesson reagent with structure shown by a formula 3; and b) performing cyclization on trifluoro ethyl hydrazine and the 2-piperazine thioketone through Pellizzari reaction and adding hydrochloric acid to form salt so as to obtain 3-trifluoromethyl-[1,2,4] triazole [4,3-a] piperazine hydrochloride serving as a target product. The method of the invention has short route, cheap and readily available raw material, high yield of each step and relatively low cost and is simple to operate.
Owner:ZHEJIANG UNIV
Sitagliptin phosphate pharmaceutical composition and preparation method thereof
ActiveCN110559270AImprove stabilityOne-sided smooth and beautifulOrganic active ingredientsMetabolism disorderAlcoholMedicine
The invention belongs to the technical field of natural medicine extraction, and particularly relates to an efficient extraction method of asparagus root saponin. The method is realized through the following steps: (1) asparagus root pretreatment; (2) primary homogenization; (3) compound enzymolysis; (4) secondary homogenization; (5) concentrating and drying; (6) extracting; and (7) alcohol precipitation. According to the method, the utilization value of the asparagus roots can be improved, and resource reutilization is achieved; the asparagus root saponin extracted by the method is high in extraction rate, and meanwhile, the prepared saponin is good in stability and high in purity.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD +1
Sitagliptin phosphate tablet and preparation method thereof
ActiveCN112494440AImprove adsorption capacityLow particle size requirementOrganic active ingredientsMetabolism disorderCombinatorial chemistryPharmaceutical Aids
The invention relates to a sitagliptin phosphate tablet. The tablet or a tablet core comprises an active component sitagliptin phosphate, filling agents, a disintegrating agent and a lubricant, and apreparation process of the sitagliptin phosphate tablet comprises following steps: the active component, the filling agents and the disintegrating agent are uniformly mixed firstly, and then the mixture is mixed with the lubricant for tabletting. According to the invention, microcrystalline cellulose and calcium sulfate are used as the filling agents, the defects of poor fluidity and easy stickingof the active component can be improved, the active component and auxiliary materials do not need to be pretreated in the preparation process, the requirement on the particle size of the material islow, the active component is not easily adsorbed to containers and utensils, and the material utilization rate is high; and when batch amplification is performed to reach 500,000 tablets / batch, no sticking phenomenon is found, the preparation process is simple, the dissolution speed is high, the product quality is excellent, the stability is good, various indexes reach the ideal level of pharmaceutics, and the sitagliptin phosphate tablet is suitable for large-scale commercial production.
Owner:JIANGSU ALPHA PHARM CO LTD
Sitagliptin pharmaceutical composition and preparation process thereof
PendingCN112843010AImprove stabilitySimple processOrganic active ingredientsMetabolism disorderActive agentPharmaceutical drug
The present invention discloses a sitagliptin pharmaceutical composition and a preparation process thereof, the sitagliptin pharmaceutical composition comprises an active agent of sitagliptin phosphate or a pharmaceutically acceptable salt thereof as an active ingredient, and by adding a pharmaceutically acceptable additive of the pharmaceutical composition, the sitagliptin pharmaceutical composition is characterized in that the granularity of 90% of the active agent is in the range of 60-300 [mu] m; the pharmaceutical composition is prepared by a direct tabletting method and coating. As for the granularity of the medicine, the stability can be effectively improved, sticking can be improved, and the problem of product degradation impurities can be controlled without grinding or preparing dry particles with other materials by a dry method or a wet method in advance. The invention also relates to a preparation method of the pharmaceutical composition, the preparation method has the characteristics of simple process, low cost, good product stability and the like, the production efficiency is improved to a certain extent, and the preparation method is suitable for commercial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Low-cost preparation method of sitagliptin phosphate
ActiveCN105061434AThe synthesis process is simpleCraft briefOrganic chemistryMetabolism disorderEpoxyZinc bromide
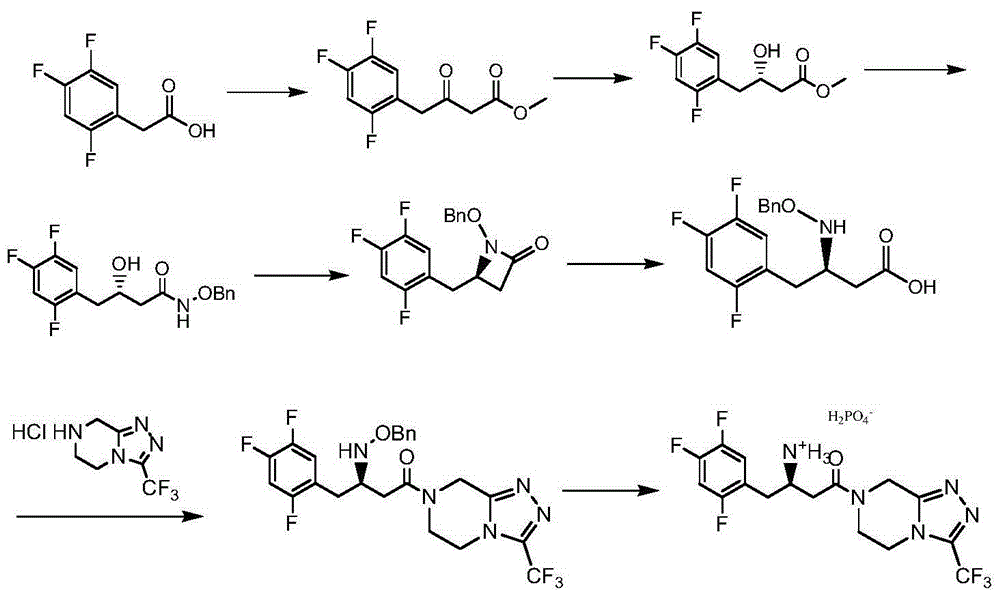
The invention relates to a low-cost preparation method of sitagliptin phosphate. The preparation method comprises the following steps: preparing N-arylmethyl-2S-cyanmethylacridine (II) from S-epoxy chloropropane or R-epoxy chloropropane; then preparing (3R)-3-arylmethylamino-4-(2,4,5-trifluorophenyl)butyric acid (III) from newly-made 2,4,5-trifluorophenyl zinc bromide and N-arylmethyl-2S-cyanmethylacridine (II) through ring-opening addition reactions, cyan basic hydrolysis, and acidification; then reacting (3R)-3-arylmethylamino-4-(2,4,5-trifluorophenyl)butyric acid (III) with an aryl-chlorination reagent to generate corresponding aryl chlorine, carrying out condensation reactions between the corresponding aryl chlorine and 3-trifluoromethyl-5,6,7,8-tetrahydro-triazole[4,3-a]pyrazine hydrochloride (IV) to prepare corresponding amide, carrying out catalytic hydrogenation to remove the aryl methyl protective groups, and acidifying the products by phosphoric acid to obtain phosphate so as to obtain sitagliptin phosphate (I). According to the preparation method, easily-available S-epoxy chloropropane or R-epoxy chloropropane is used to prepare N-arylmethyl-2S-cyanmethylacridine (II) so as to construct a chiral center of the sitagliptin phosphate; the reaction conditions are mild, the operation is easy, the sitagliptin phosphate yield is high, the impurities are little, and the cost is low.
Owner:XINFA PHARMA
Sitagliptin phosphate tablet and preparation method thereof
InactiveCN113712930AFast dissolution rateGood hypoglycemic effectOrganic active ingredientsMetabolism disorderSitagliptin PhosphateBlood sugar
The invention relates to the technical field of medicine production, in particular to a sitagliptin phosphate tablet and a preparation method thereof. The sitagliptin phosphate tablet is prepared from the following raw materials in parts by mass: 30 parts of sitagliptin phosphate, 40-80 parts of a filling agent, 2-5 parts of a disintegrating agent and 10-20 parts of a lubricating agent. Compared with the prior art, through the measurement of the dissolution rate, the sitagliptin phosphate tablet prepared by adopting the formula and the two methods has the dissolution rate of more than 70% in 900ml of water medium (slurry method, 50rpm) in 5minutes, the dissolution rate is higher, and the blood sugar reducing effect is good; and the preparation process is simple, the reproducibility is good, and the industrial production condition is provided.
Owner:SHANDONG RENHE PHARMA
Preparation method of unknown impurity of sitagliptin phosphate tablet
InactiveCN110590783AQuality is easy to controlGuaranteed expected efficacyOrganic chemistryMaterial analysis by electric/magnetic meansOrganic synthesisPhosphate
The invention discloses a preparation method of an unknown impurity of a sitagliptin phosphate tablet, and relates to the technical field of organic synthesis. Sitagliptin and maleic anhydride are taken as raw materials to carry out an addition reaction under action of a catalyst to obtain the sitagliptin impurity. The preparation method can be used for efficiently synthesizing the sitagliptin impurity (I), and the structure of the impurity is characterized and determined by nuclear magnetism and mass spectrum; and the impurity is taken as a reference substance to qualitatively and quantitatively analyze whether impurities are generated in a processing or placing process of a siagliptin phosphate preparation and qualitatively and quantitatively analyze the content of the impurities in thesiagliptin phosphate preparation, so that quality control of the siagliptin phosphate preparation can be facilitated and an expected drug effect of the siagliptin phosphate preparation is ensured.
Owner:HEFEI HUAFANG PHARMA SCI & TECH +1
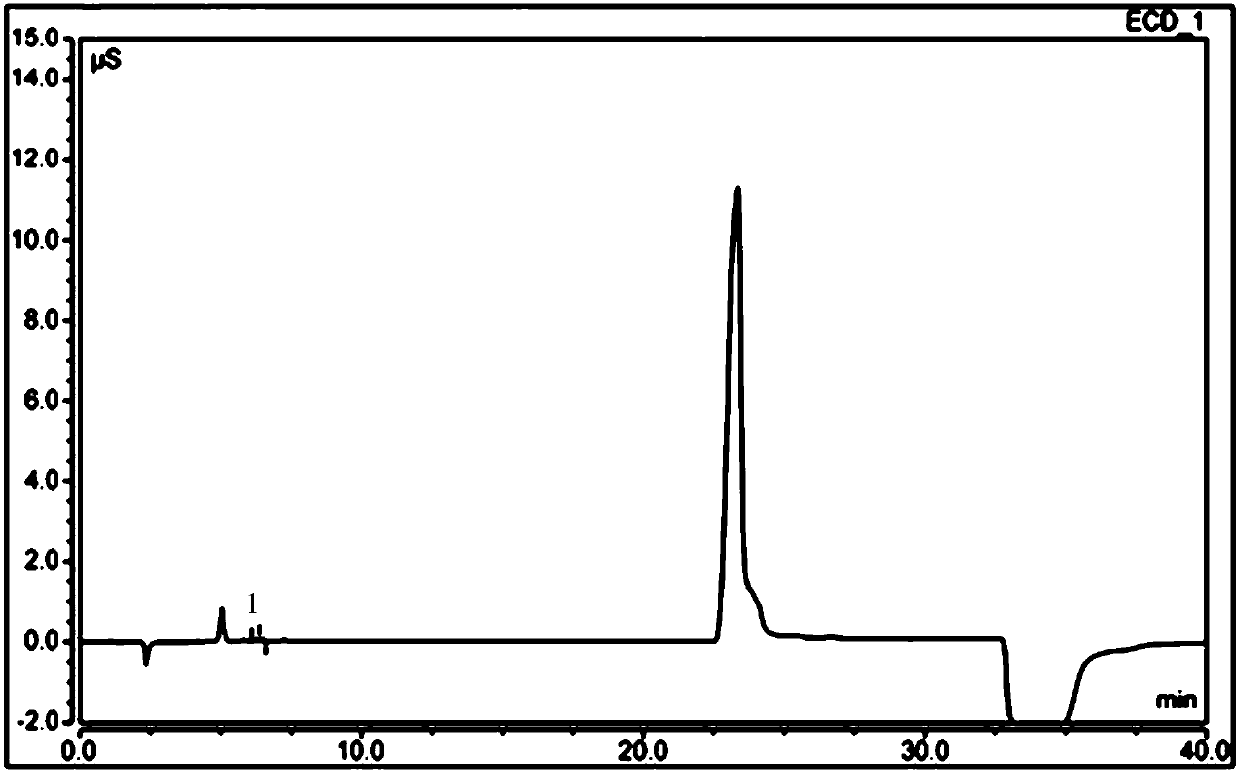
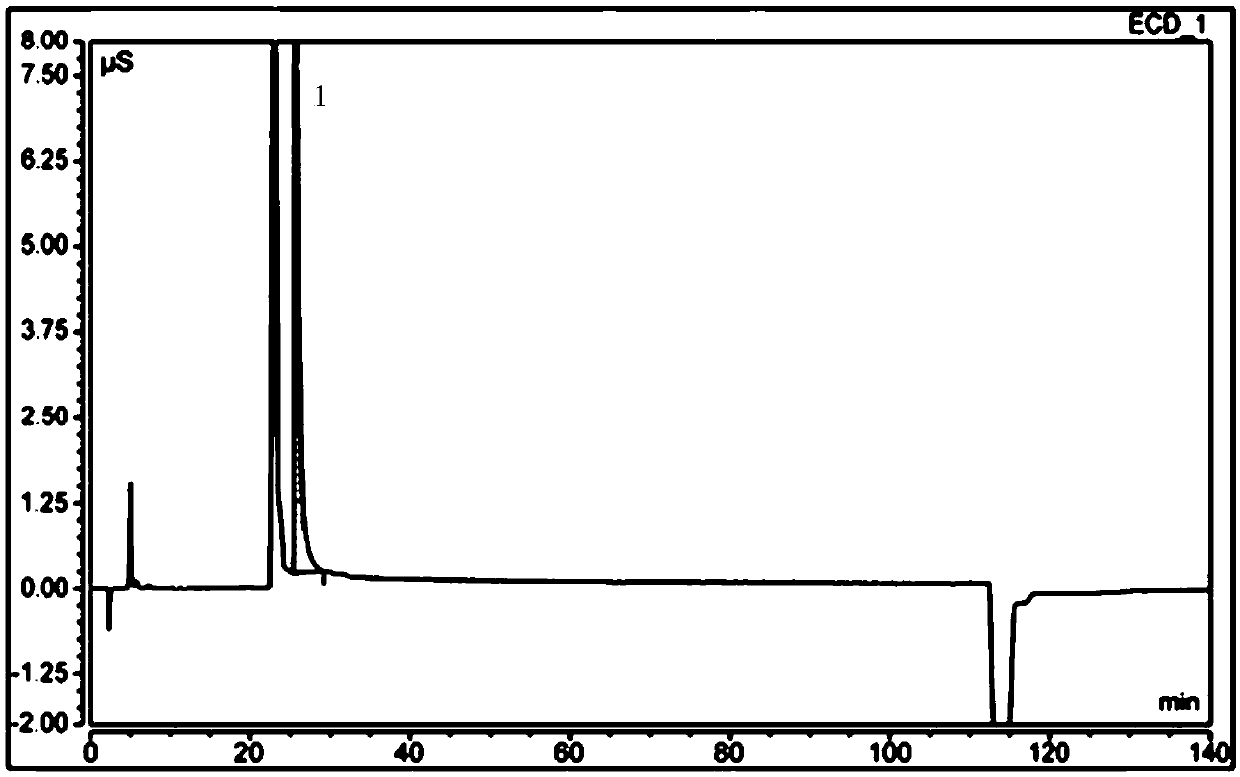
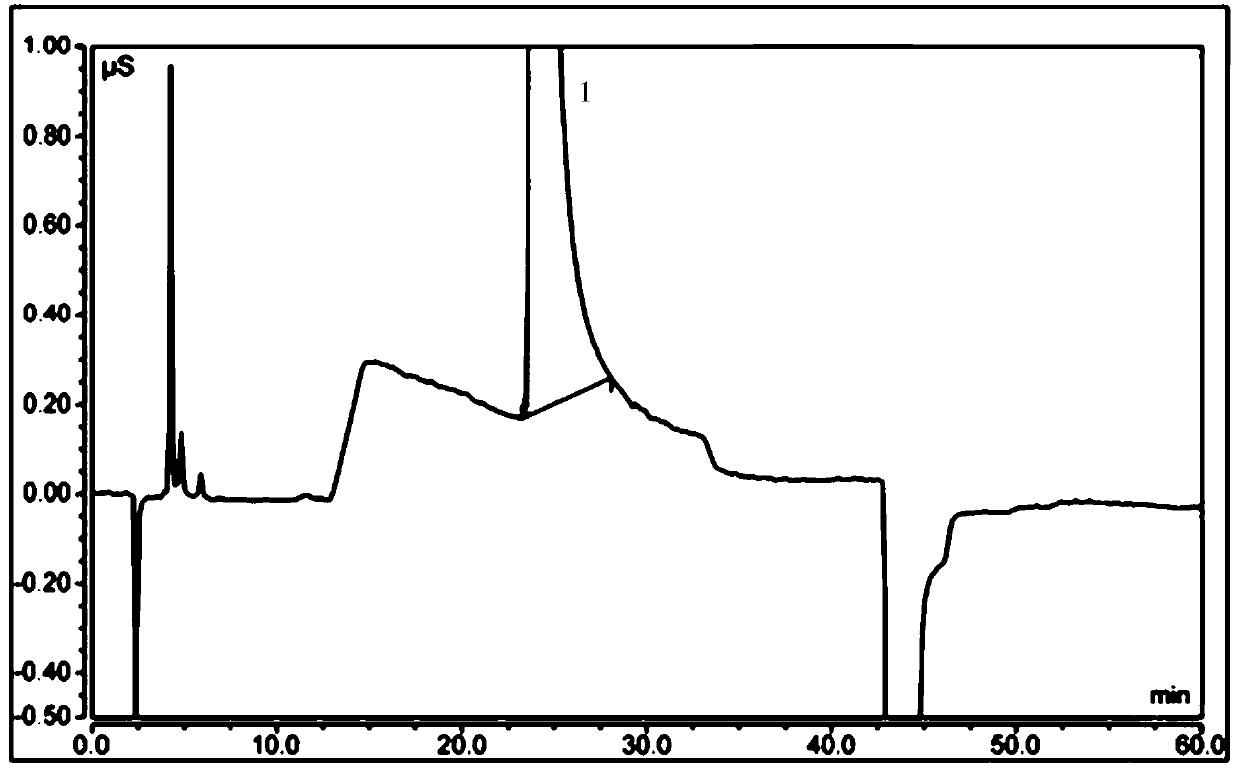
Free hydrazine detection method and application thereof in sitagliptin phosphate bulk drug impurity detection
InactiveCN109682921AQuick checkEfficient detectionComponent separationIon chromatographyHydrazine compound
The invention provides a detection method for free hydrazine on the basis of ion chromatography. According to the chromatographic conditions, a chromatography column is a Thermo Dionex IonPac CS12A chromatography column with the specification being 250mm*4mm; a protective column is a Thermo Dionex IonPac CS12A chromatography column with the specification being 4 mm*50mm; the specification of a positive ion inhibitor CERS is 4 mm; a detector is an electric conduction detector; a moving phase is 5 mmol / l loprazolam; the flow speed is 0.8-1.3 ml / min; the column temperature is 28-35 DEG C; the sampling size is 25 microliters. According to the detection method, the minimum linear detection concentration is 0.18 microgram / ml, and the minimum detection concentration is 0.05 microgram / ml. The invention further provides application of the detection method in sitagliptin phosphate bulk drug impurity detection.
Owner:TONGHUA DONGBAO PHARMA
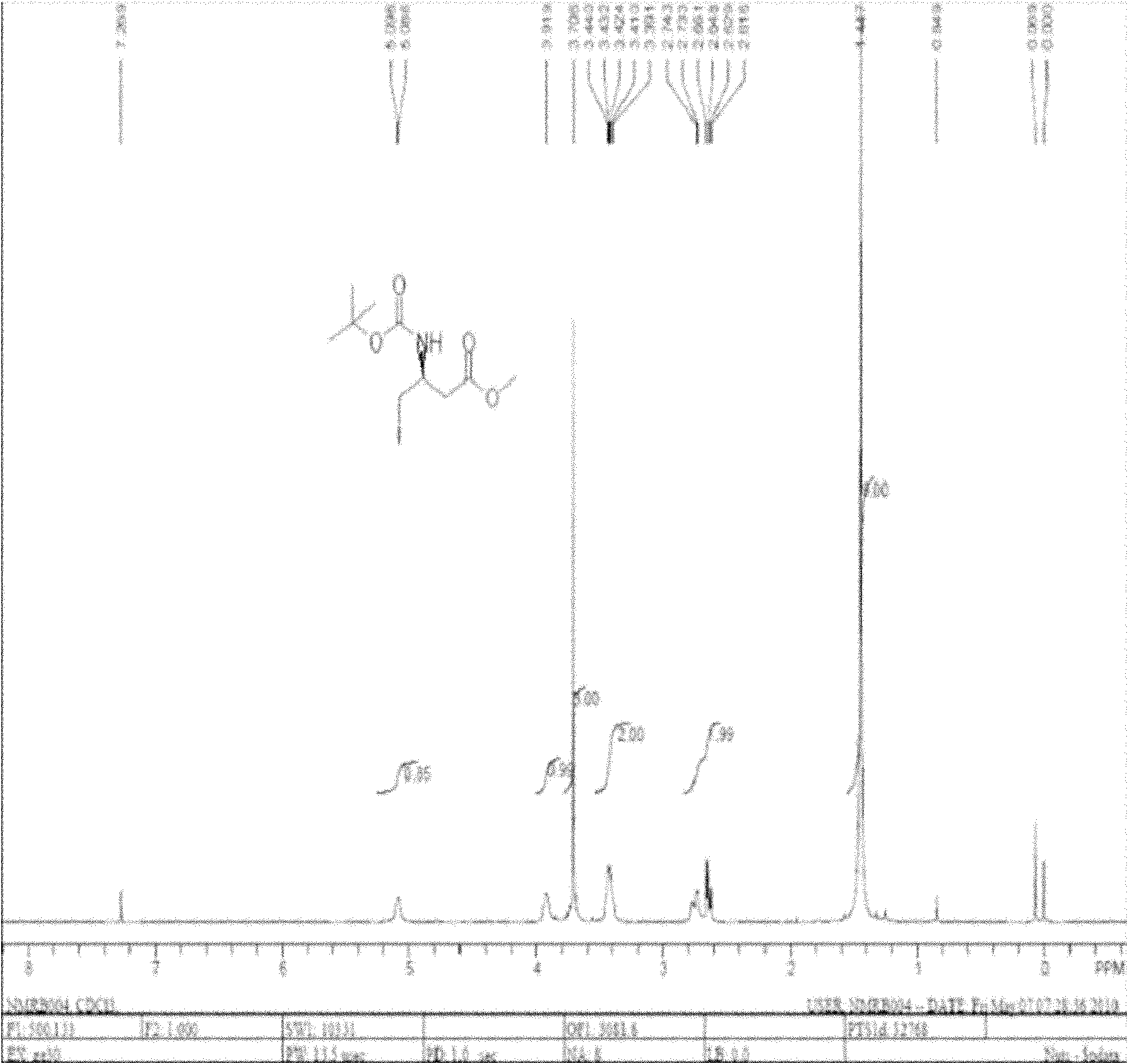
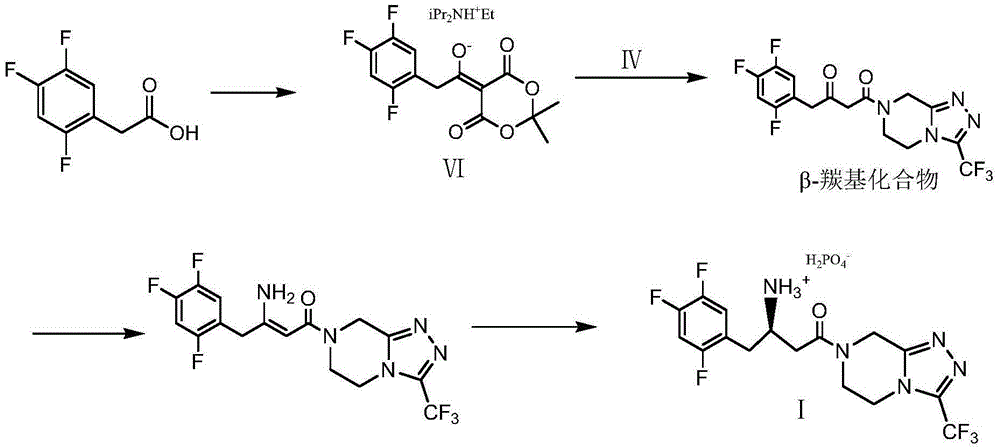
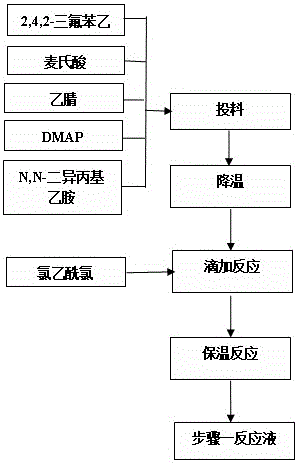
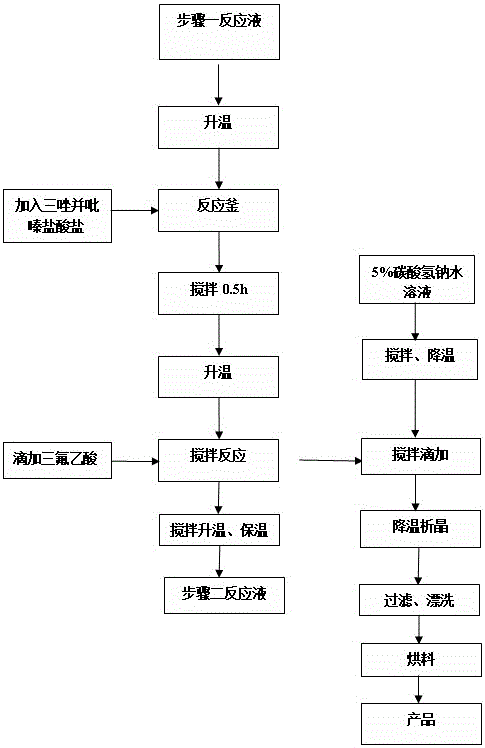
Synthesis method of sitagliptin phosphate intermediate
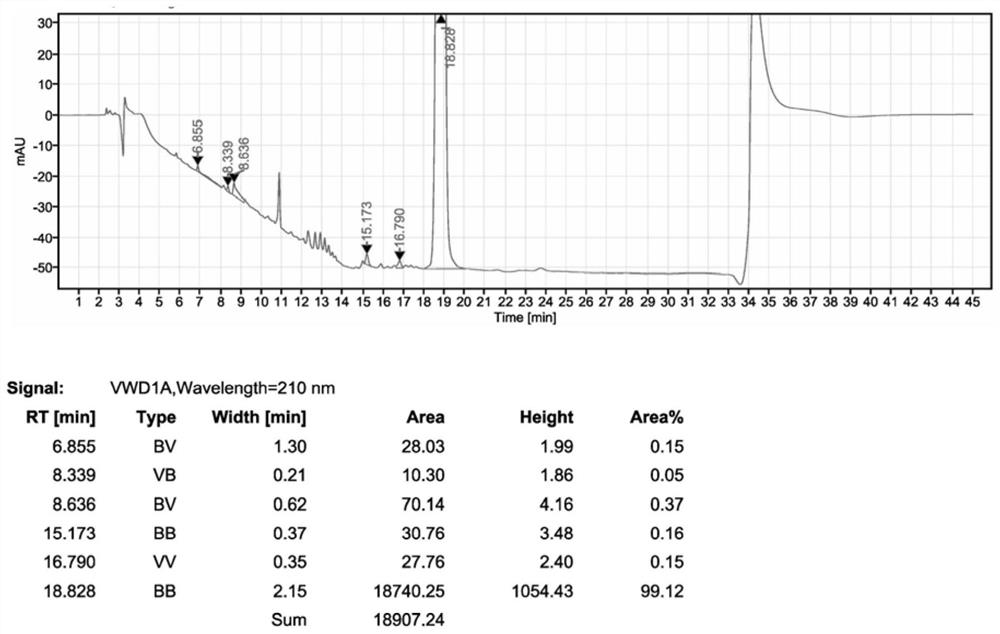
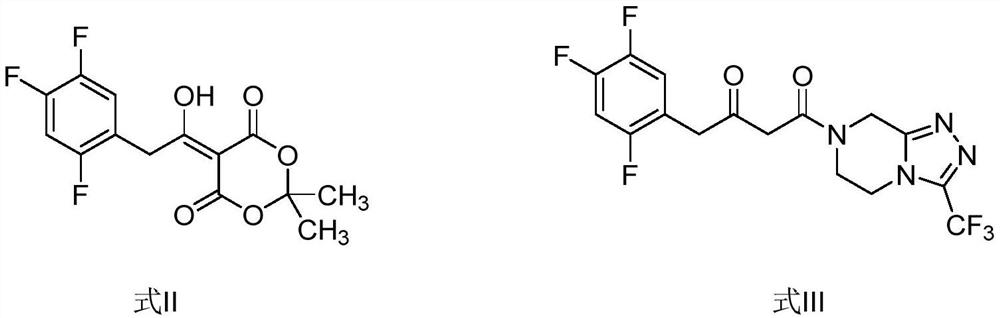
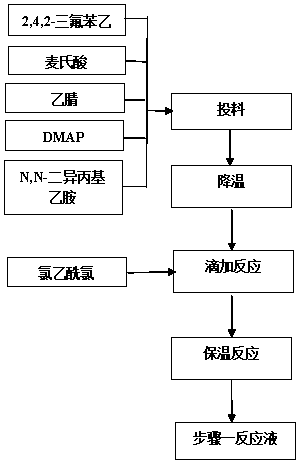
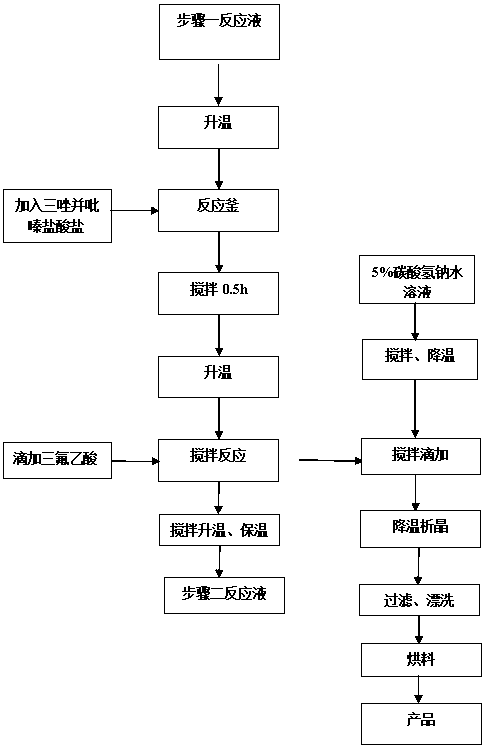
The invention relates to a synthesis method of a sitagliptin phosphate intermediate, and belongs to the technical field of organic synthesis. The method comprises the following technological steps of synthesizing a reaction liquid of 5-hydroxyl-[(2,4,5-trifluoro-phenyl)-ethylidene]-dimethyl-[1,3] dioxo-4,6-diketone from 2,4,5-trifluoro-phenylacetic acid, meldrum's acid, N,N-diisopropylethylamine, DMAP and acetylchloride; adding 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4] triazol [4,3-alpha] pyrazine hydrochloride and trifluoroacetic acid to obtain the product. According to the method, the reaction raw materials are easily available; the reaction process is simple in operation; the requirements on reaction equipment are low; the reaction conditions are relatively mild; the yield and content are high, the quantity of waste water and solid wastes is relatively low, the cost is saved, and the method is suitable for industrial production.
Owner:SULI PHARMA TECH JIANGYIN
Novel method for synthesizing sitagliptin phosphate and derivative thereof
InactiveCN102153559BRaw materials are easy to getReduce manufacturing costOrganic chemistryBulk chemical productionChemical reactionL-Aspartate
The invention discloses a novel method for synthesizing sitagliptin phosphate and a derivative thereof. The method has the advantages of readily-available raw materials, mild reaction condition, no need of using very expensive catalyst, easy control over optical purity and the like. In the novel method for synthesizing sitagliptin phosphate and the derivative thereof, L-aspartic acid is taken as a raw material, and the chemical reaction steps of amino protection, esterification, reduction, iodination or bromination, coupling, hydrolysis, acylation ammoniation, removal of protecting groups andsalt formation are performed to obtain a sitagliptin phosphate chiral salt and a hydrate thereof. The method comprises the following specific steps of: performing amino protection, esterification, reduction and iodination on L-aspartic acid serving as the raw material to obtain a compound A shown as a formula I; undergoing a coupling reaction on the compound A and a compound B shown as a formula II to obtain a compound C shown as a formula III; and performing hydrolysis, acylation ammoniation, removal of protecting groups and a salt-forming reaction on the compound C to obtain a sitagliptin phosphate hydrate.
Owner:NANJING TECH UNIV
Synthetic method of sitagliptin phosphate impurities
InactiveCN108863837ALower requirementEasy to operateOrganic compound preparationOrganic chemistry methodsHydrolysisSitagliptin Phosphate
The invention discloses a synthetic method of sitagliptin phosphate impurities. The synthetic method is characterized in that the sitagliptin phosphate impurities are (R)-3-(S)-2-amino-2-oxo-1-phenethyl ammonia-4-(2,4,5-fluorophenyl) butanoic acid and are obtained by taking beta-keto ester, S-phenylglycine amide and glacial acetic acid as starting materials through a three-step technology of Schiff base reaction, hydrogenation reaction and hydrolysis reaction. The synthetic method provided by the invention is relatively available in reaction raw materials, simple to operate in reaction process, low in requirement for reaction equipment, relatively moderate in reaction condition and high in yield and content, saves the cost and can have very great promotion effect to deeper and wider studyon the drug use safety, the reliability and the stability relative to sitagliptin phosphate and quality control in the production process.
Owner:SULI PHARMA TECH JIANGYIN
Preparation method of sitagliptin phosphate intermediate
The invention discloses a preparation method of a sitagliptin phosphate intermediate. In the existing synthesis method of the sitagliptin phosphate intermediate, a corresponding post-treatment method is not reported or the reported post-treatment method is tedious in operation, low in solvent recovery rate, capable of generating a large amount of three wastes, and not beneficial to environmental protection. The method comprises the following steps: directly evaporating to remove a solvent after the synthesis reaction of the sitagliptin phosphate intermediate is completed, adding a crystallization solvent, cooling, stirring for crystallization, washing and drying to obtain the corresponding sitagliptin phosphate intermediate. According to the invention, the yield and the purity of the sitagliptin phosphate intermediate are improved; the method is simple in process, high in solvent recovery rate and suitable for large-scale industrial production, and wastewater is not generated in the production process.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY +1
A kind of synthetic method of sitagliptin phosphate intermediate
Owner:SULI PHARMA TECH JIANGYIN
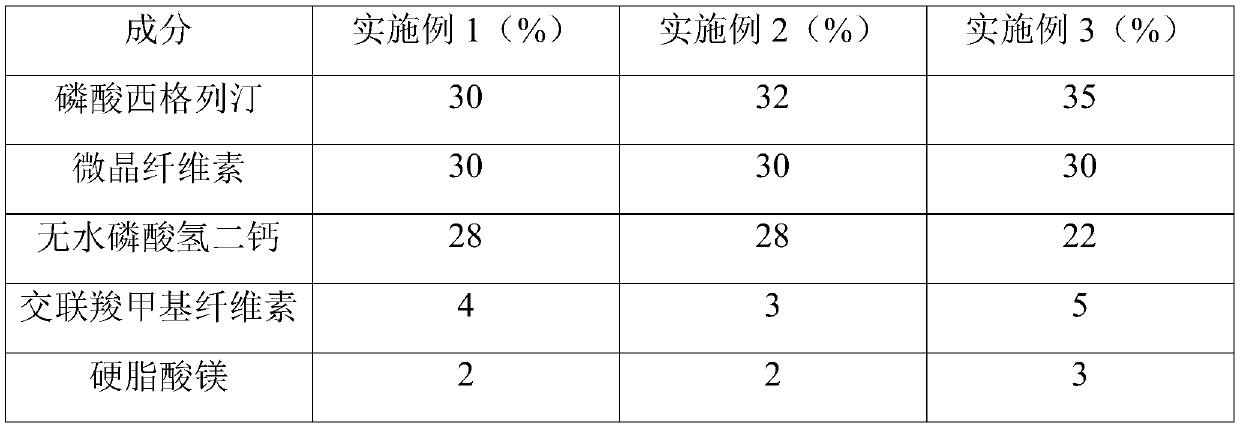
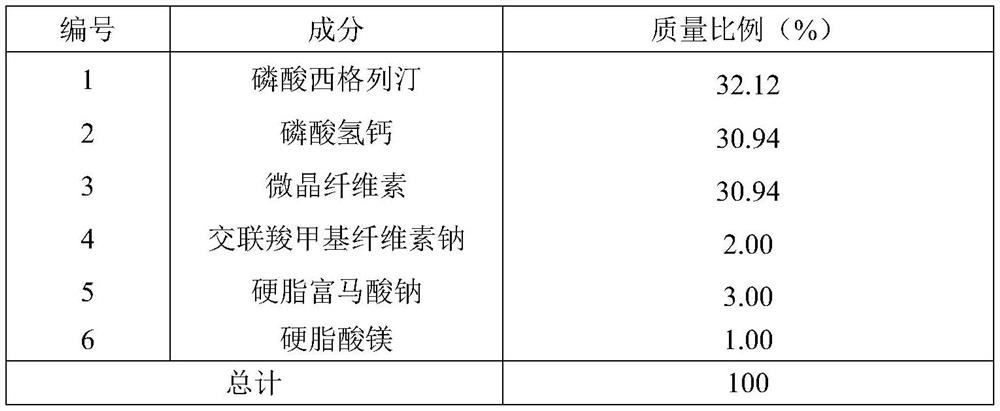
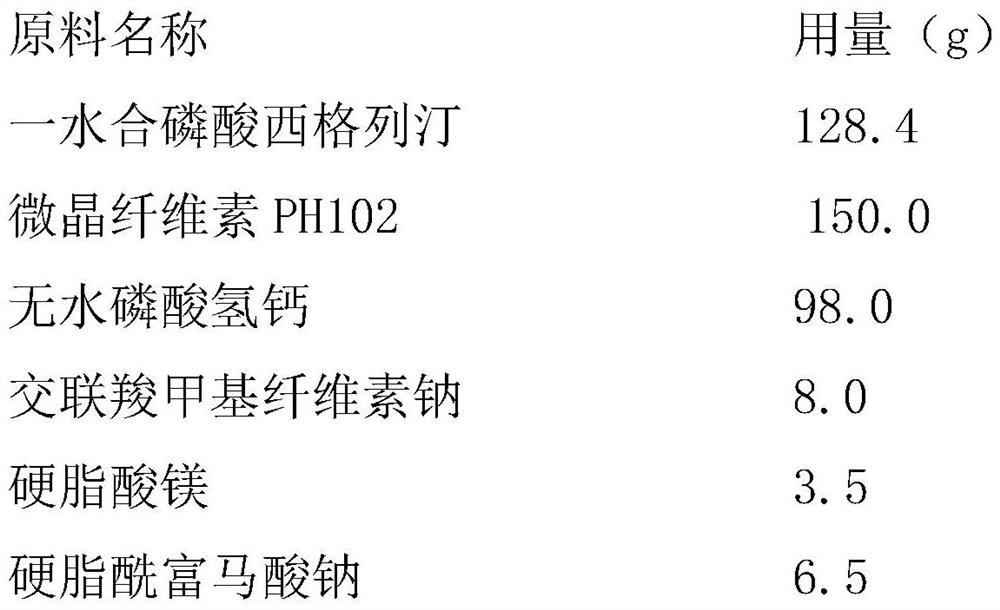
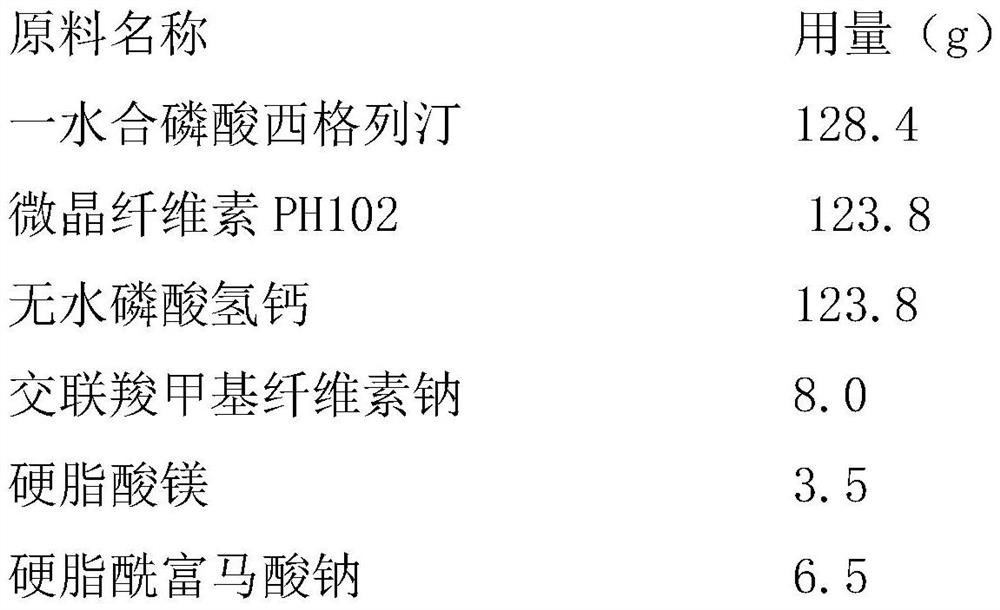
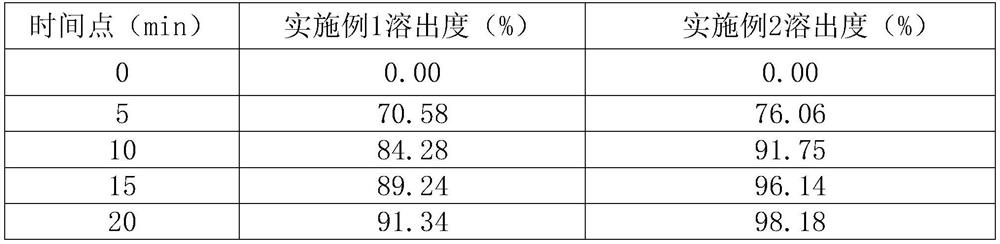
Dry granulation process of sitagliptin phosphate composition
ActiveCN112206210BImprove stabilityOrganic active ingredientsMetabolism disorderHydrogen phosphatePhosphate
The invention discloses a dry granulation process for a sitagliptin phosphate composition, which includes the following steps: 1) selecting a phosphate liptin bulk drug with needle-like or rod-like shape; 2) premixing microcrystalline cellulose, west phosphate Gliptin and anhydrous calcium hydrogen phosphate are mixed and sieved, and then sieved croscarmellose sodium and sodium stearyl fumarate are added for blending; 3) granulation, and the final step in step 2). The mixture is prepared into granules by a dry granulator; 4) The material obtained from granulation is mixed with the sieved magnesium stearate. The needle-like or rod-like crystal habit has a larger specific surface area and is more prone to API inconsistencies. In a stable situation, with the addition of sodium stearyl fumarate in the granules, it is beneficial to accelerate the disintegration rate of the tablet. After the dry granules are prepared, most of sitagliptin phosphate has been wrapped in the dry granules, and then When mixed with magnesium stearate, the contact area between API and magnesium stearate is greatly reduced, ensuring the stability of the finished product.
Owner:宁波高新区美诺华医药创新研究院有限公司
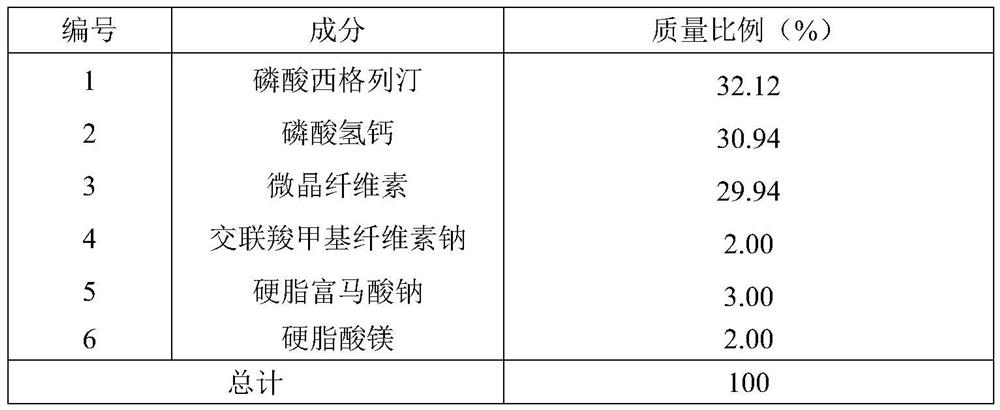
Sitagliptin phosphate composition, sitagliptin phosphate tablet, and preparation method and application of sitagliptin phosphate composition and sitagliptin phosphate tablet
ActiveCN113679684ASolve the problem of inconsistent dissolution in vitroOrganic active ingredientsMetabolism disorderSodium fumarateStearic acid
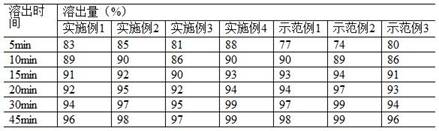
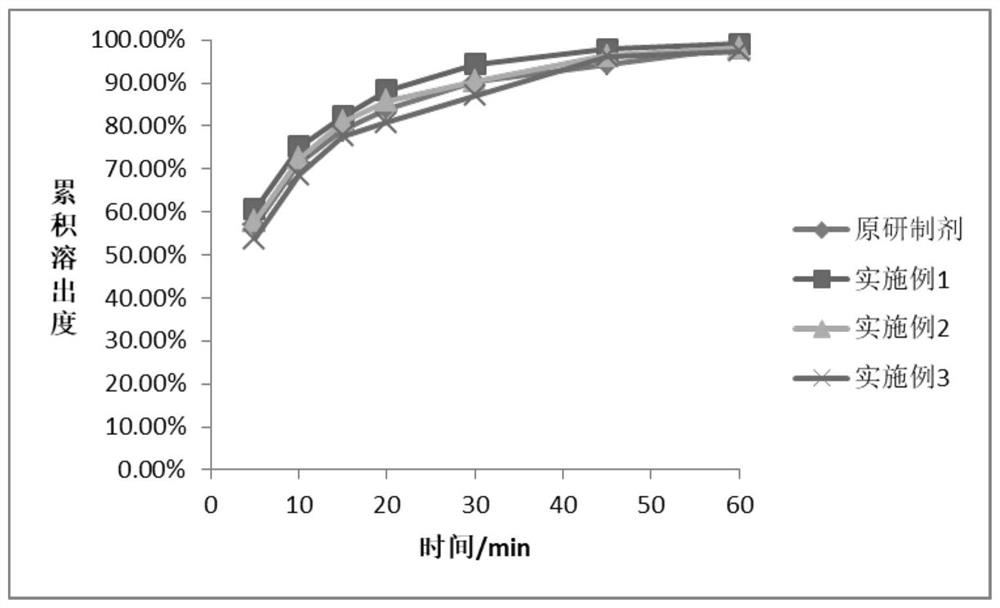
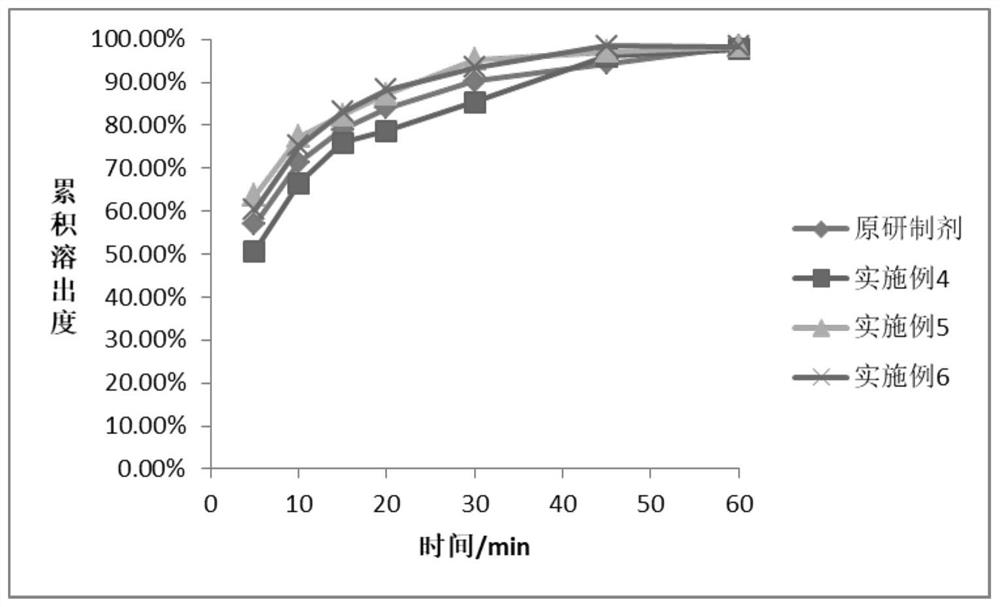
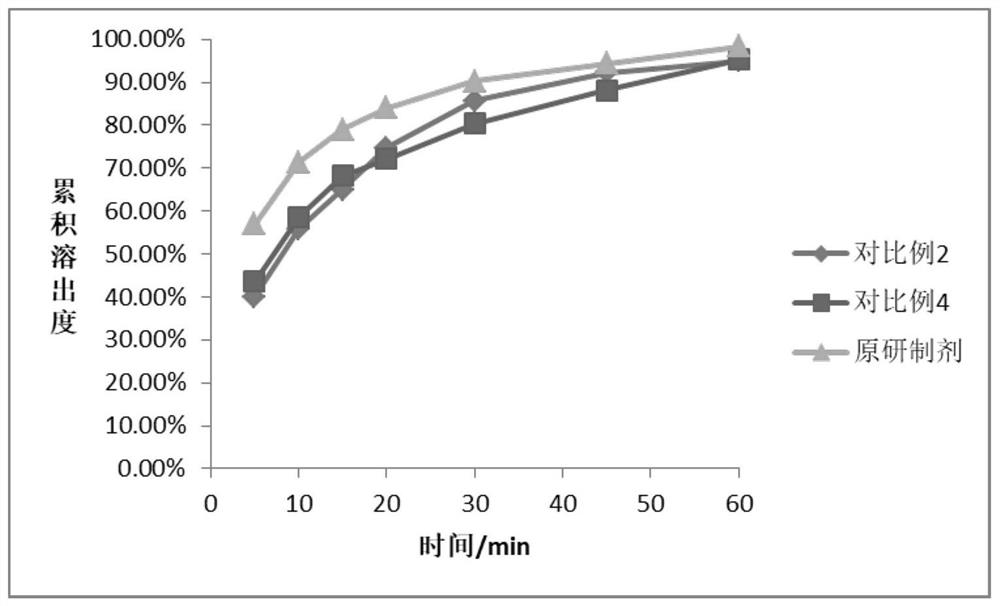
The invention belongs to the field of medicines, and particularly relates to a sitagliptin phosphate composition, sitagliptin phosphate tablets and a preparation method and application of the sitagliptin phosphate composition and the sitagliptin phosphate tablets, and the sitagliptin phosphate composition comprises the following components in percentage by mass: 25-35% of sitagliptin phosphate monohydrate; 20%-40% of anhydrous calcium hydrogen phosphate; 20%-40% of microcrystalline cellulose; 1%-4% of croscarmellose sodium; 0.5%-2% of magnesium stearate; and 1%-4% of sodium stearyl fumarate, wherein the D90 particle size of the sitagliptin phosphate monohydrate is 30-90 [mu]m, and the D90 particle size of the microcrystalline cellulose is greater than or equal to 260 [mu]m. The in-vitro dissolution curve of the sitagliptin phosphate tablet provided by the invention is similar to that of an imported original development agent produced by Merk Company, so that the possibility of bioequivalence with the original development agent is improved, and the requirement of consistency evaluation is met.
Owner:北京鑫开元医药科技有限公司
Derivatization treatment method for drug enantiomer detection, determination method and application
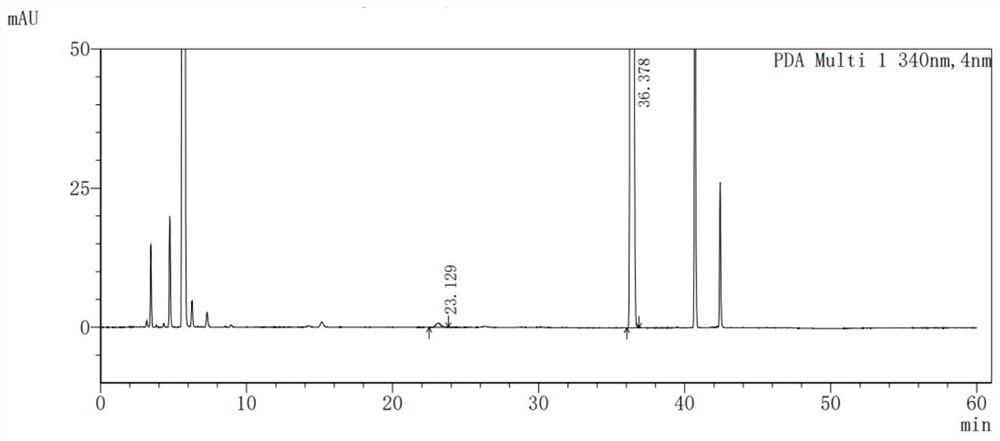
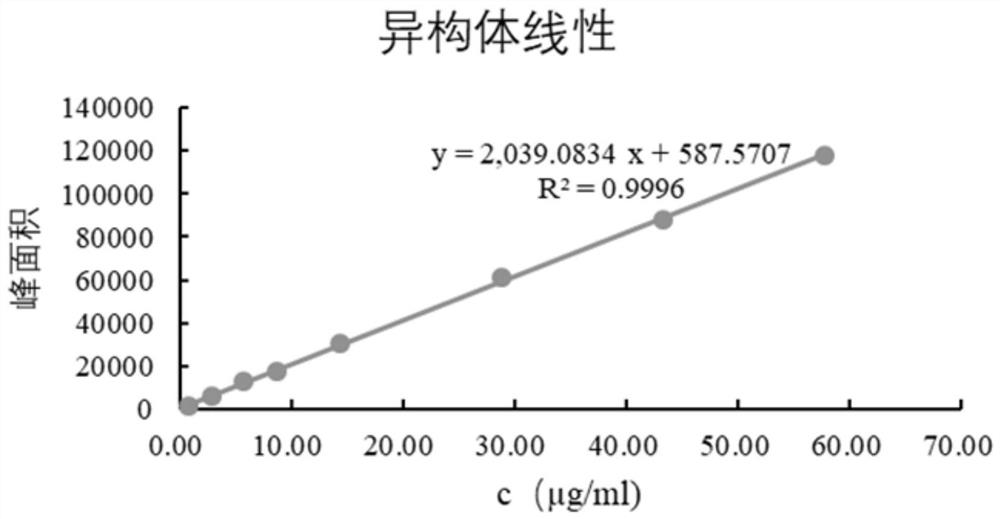
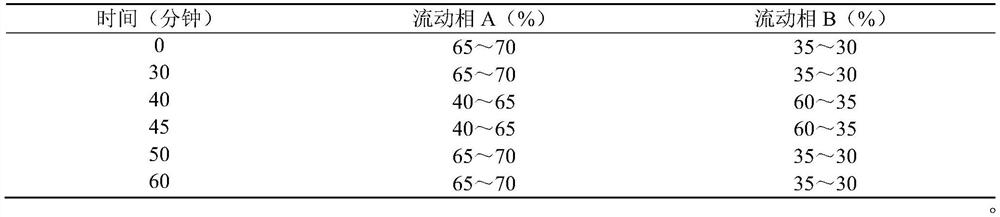
The invention discloses a derivatization treatment method for drug enantiomer detection, a determination method and application. When the derivatization treatment method is used for detecting isomers in sitagliptin phosphate bulk drugs and preparations, high performance liquid chromatography is adopted for detection, and chromatographic conditions are as follows: octadecylsilane chemically bonded silica is used as a filler, a triethylamine aqueous solution is used as a mobile phase A and acetonitrile is used as a mobile phase B for gradient elution, the flow velocity is 1.5 ml per minute, and the detection wavelength is 340 nm; and a sample is prepared by adopting a reverse phase system for dissolving, and derivatization treatment is carried out by adopting a Marfeed reagent. The sitagliptin phosphate raw material medicine and enantiomer have high separation degree, the problem that an existing detection technology is long in test sample preparation time, poor in reproducibility and high in cost can be solved, and the method has the advantages of strong specificity, strong accuracy, high precision, good durability and convenience of operation, and can effectively control the quality of bulk drugs and preparations in actual production.
Owner:SICHUAN PHARMA
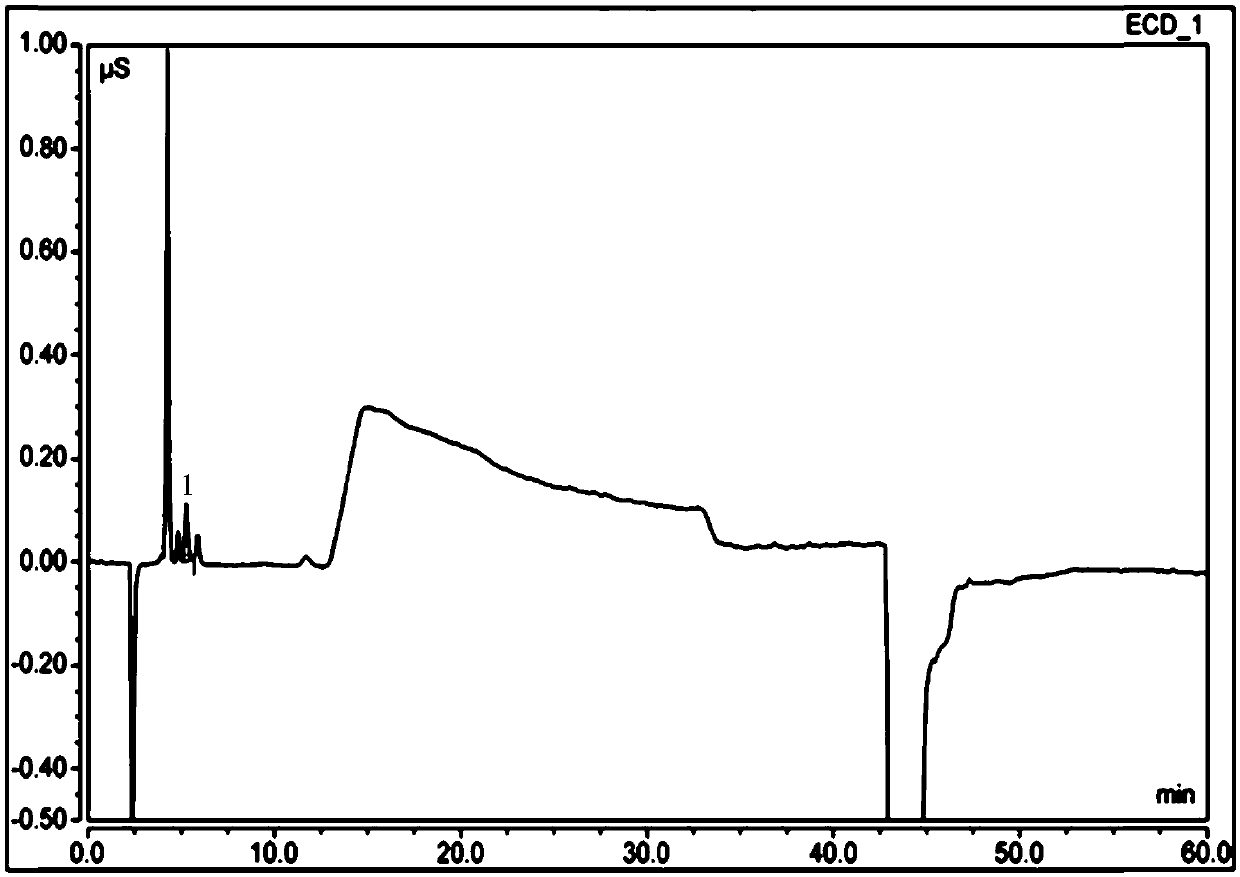
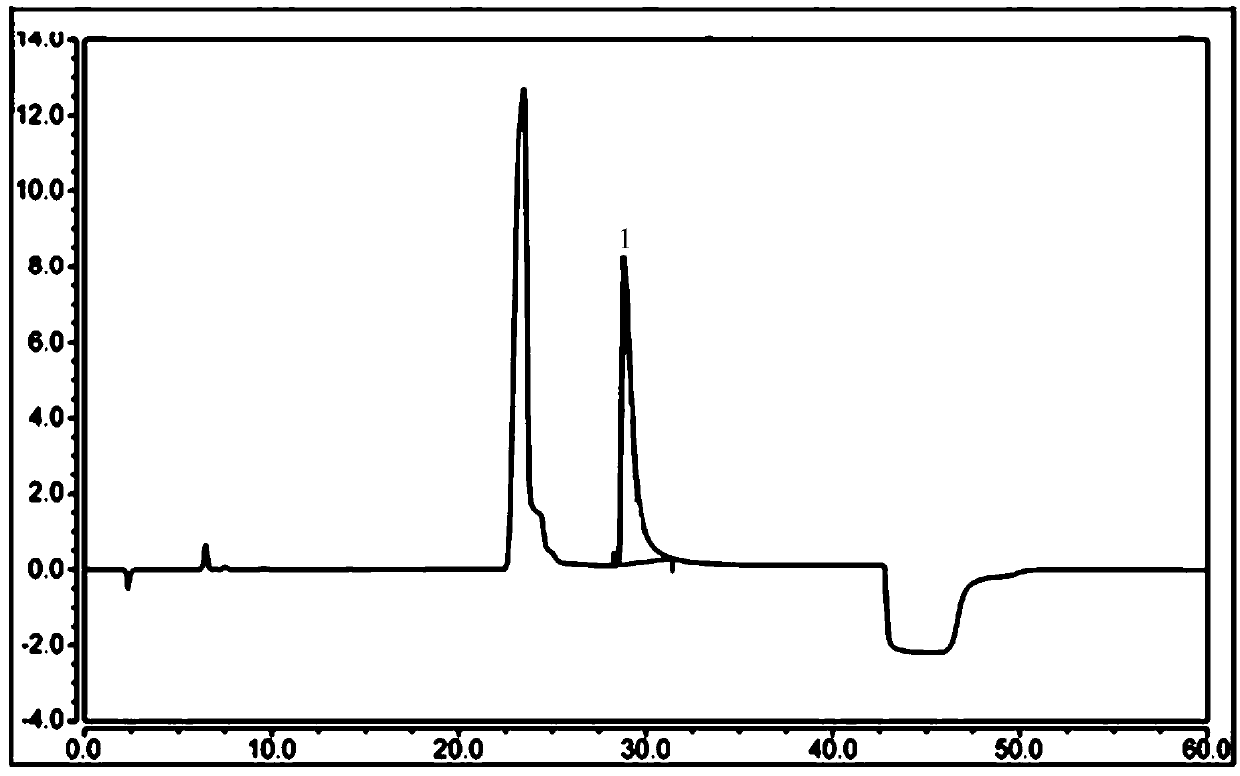
Detecting method of ethene diamine and method for detecting ethene diamine in sitagliptin phosphate intermediate XG-SM2
ActiveCN109655539AEfficient determinationRapid determinationComponent separationMethane sulfonic acidEthylenediamine
The invention provides a detecting method of ethene diamine based on ion chromatography. A chromatogram condition is characterized in that a chromatographic column is a Thermo Dionex IonPac CS12A cation chromatographic column, 250mm*4mm; a detector is a conductivity detector; a mobile phase is 40mmol / l methane sulfonic acid; the flow velocity is 0.7ml / min - 1.4ml / min; the column temperature is 28-35 DEG C; and the sample entering amount is 25 ul. The lowest linear detection concentration of the detecting method is 0.4[mu]g / ml. The lowest detection concentration of the method with a signal-to-noise ratio of 3:1 is 0.06 [mu]g / ml. The invention further provides application of the detecting method in sitagliptin phosphate intermediate XG-SM2 impurity detection.
Owner:TONGHUA DONGBAO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com