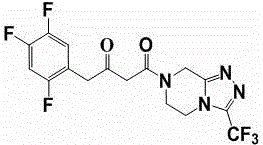
Synthesis method of sitagliptin phosphate intermediate
A technique for the synthesis of sitagliptin phosphate, which is applied in the field of organic synthesis, can solve the problems of difficult recovery of solvent N,N-dimethylacetamide, high cost of pivaloyl chloride, large amount of waste solid waste water, etc. The effect of low equipment requirements, low cost, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
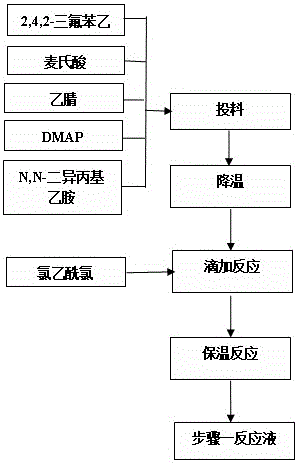
[0029] Step 1. Condensation
[0030] Add 3kg of 2,4,5-trifluorophenylacetic acid, 2.5kg of McBurney's acid, 4.18kg of N,N-diisopropylethylamine, and 0.154kg of DMAP into organic solvent A, protect with nitrogen, and cool down to -10~0 After dropping 1.5kg of acetyl chloride, drop it for about 2 hours, then raise the temperature to 0°C for 5 hours, then raise the temperature to 30~40°C to obtain 5-hydroxy-[(2,4,5-trifluoro The reaction solution of phenyl)-ethylene]-dimethyl-[1,3]dioxy-4,6-dione is directly used for the next step reaction without discharging.
[0031] Step 2, open loop
[0032] Add 3.6 kg of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-α]pyrazine hydrochloride into Step 1 at one time In the reaction solution, after stirring for 0.5h at a temperature of 30~40°C, start to add 0.54kg of trifluoroacetic acid dropwise, after about 1h, the temperature is raised to 55~60°C, and the reaction is kept for 6~8h. Add the reaction solution dropwise to 18L of...
Embodiment 2
[0034] Step 1. Condensation
[0035] With embodiment 1.
[0036] Step 2, open loop
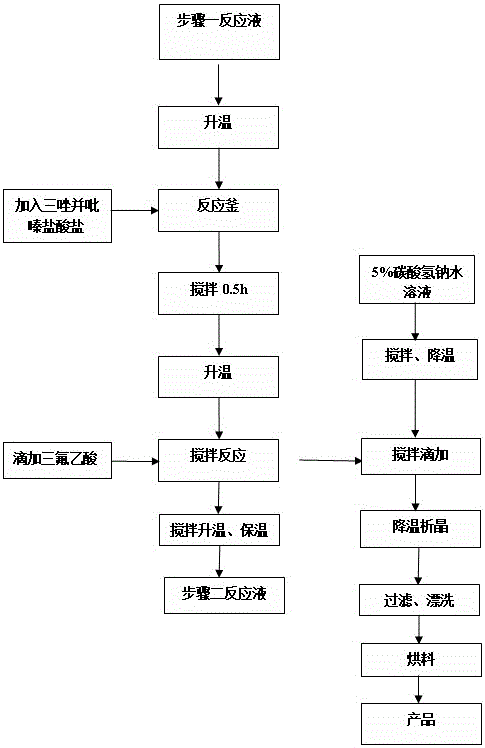
[0037] Add 3.6 kg of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-α]pyrazine hydrochloride into Step 1 at one time In the reaction solution, after stirring for 0.5h at a temperature of 30~40°C, start to add 0.54kg of trifluoroacetic acid dropwise, after about 1h, the temperature is raised to 55~60°C, and the reaction is kept for 6~8h. Add the reaction solution dropwise to 18L of aqueous solution containing 5% sodium bicarbonate, control the temperature at 10-15°C, and finish the dripping in about 3 hours. After suction filtration, rinse the filter cake with 1.5L of mixed solvent (acetonitrile: water = 6:1), and send the filter cake to a decompression oven for drying (control temperature 50~55°C, vacuum degree ≥ 0.085MPa) to obtain ( 2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7-( 8H)-yl]-1-(2,4,5-,trifluorophenyl)butan-2-one solid dry product 5...
Embodiment 3
[0039] Step 1. Condensation
[0040] With embodiment 1.
[0041] Step 2, open loop
[0042]Add 3.6 kg of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-α]pyrazine hydrochloride into Step 1 at one time In the reaction solution, after stirring for 0.5h at a temperature of 30~40°C, start to add 0.54kg of trifluoroacetic acid dropwise, after about 1h, the temperature is raised to 55~60°C, and the reaction is kept for 6~8h. Add the reaction solution dropwise to 18L of aqueous solution containing 5% sodium bicarbonate, control the temperature at 10-15°C, and finish the dripping in about 3 hours. After suction filtration, rinse the filter cake with 1.5L of mixed solvent (acetonitrile: water = 6:1), and send the filter cake to a decompression oven for drying (control temperature 50~55°C, vacuum degree ≥ 0.085MPa) to obtain ( 2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7-( 8H)-yl]-1-(2,4,5-,trifluorophenyl)butan-2-one solid dry product 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com