Sitagliptin phosphate impurities, method for preparing same and application of sitagliptin phosphate impurities
A technology of sitagliptin phosphate and sitagliptin impurities, which is applied in the field of drug synthesis to achieve the effects of strong safety, simple and efficient operation, and moderate reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
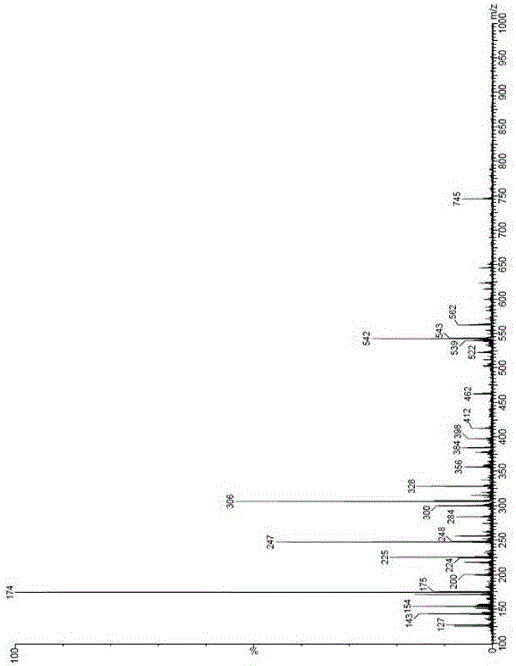
[0056] Example 1 Preparation of sitagliptin phosphate impurity A
[0057] Add (R)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (SM1, 23.3g, 0.1mol) into 250ml of dichloromethane at room temperature, and then add di-tert-butyl dicarbonate Esters (Boc anhydride, 98.22g, 0.45mol) and triethylamine (100g, 0.99mol) were stirred and reacted at room temperature for 5 hours, the reaction system was concentrated, and the concentrate was purified by silica gel column (dichloromethane:methanol volume ratio: 20:1) , the eluate containing impurity A was collected and concentrated to dryness to obtain a white solid (impurity A, 26 g, 60%). The purity is 97.84%.
[0058] 1 H-NMR (600HZ, DMSO-d6): δ7.49 (m , 1H), 7.45 (m , 1H), 6.83 (d , 1H), 4.01 (s, 1H), 2.82 (m , 1H), 2.41( m, 1H), 2.33 (m, 1H), 1.39 (s, 9H), 1.27 (s, 9H). MS (m / z): 433.42. [M-C4H9 / +K] + :412.
Embodiment 2
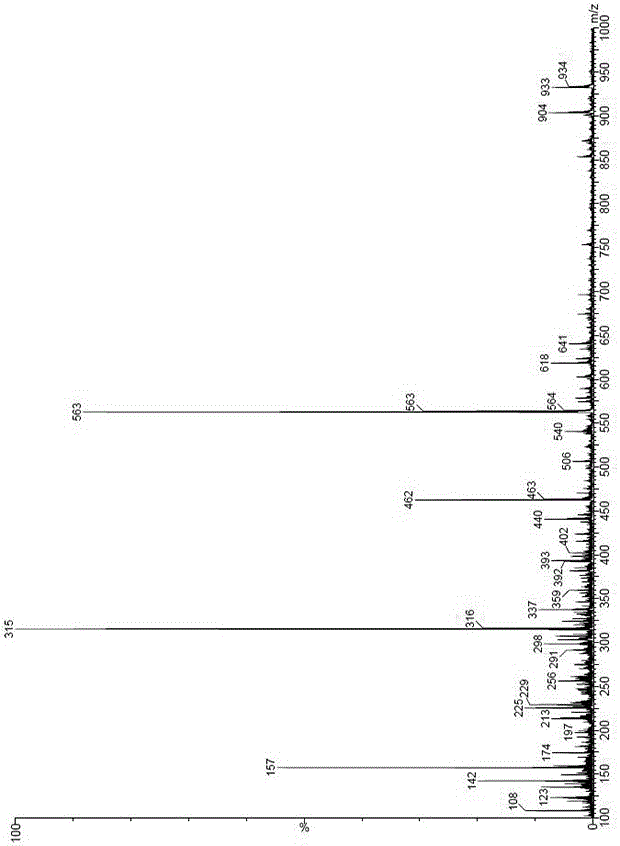
[0059] The preparation of embodiment 2 sitagliptin phosphate impurity B
[0060] 1. Preparation of Compound II
[0061] Add (R)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (SM1, 46.6g, 0.20mol) into 500ml of dichloromethane at room temperature, and then add di-tert-butyl dicarbonate Esters (Boc anhydride, 43.65 g, 0.2 mol) and triethylamine (60.71 g, 0.60 mol) were stirred and reacted at room temperature for 3 hours. Add 100ml of 5% dilute hydrochloric acid to the system to wash, then wash the organic layer with water, separate the layers, wash the organic layer with saturated brine, dry over anhydrous magnesium sulfate, filter, concentrate the filtrate to dryness, add 200ml of n-hexane to crystallize to obtain a white solid (i.e. Compound II, 56.7 g, 85%).
[0062] , Preparation of Sitagliptin Phosphate Impurity B
[0063] Compound II (35g, 0.11mol), N,N-dicyclohexylcarbodiimide (DCC, 74.22g, 0.36mol) and triethylamine (33.39g, 0.33mol) were added to 250ml N,N- In dime...
Embodiment 3
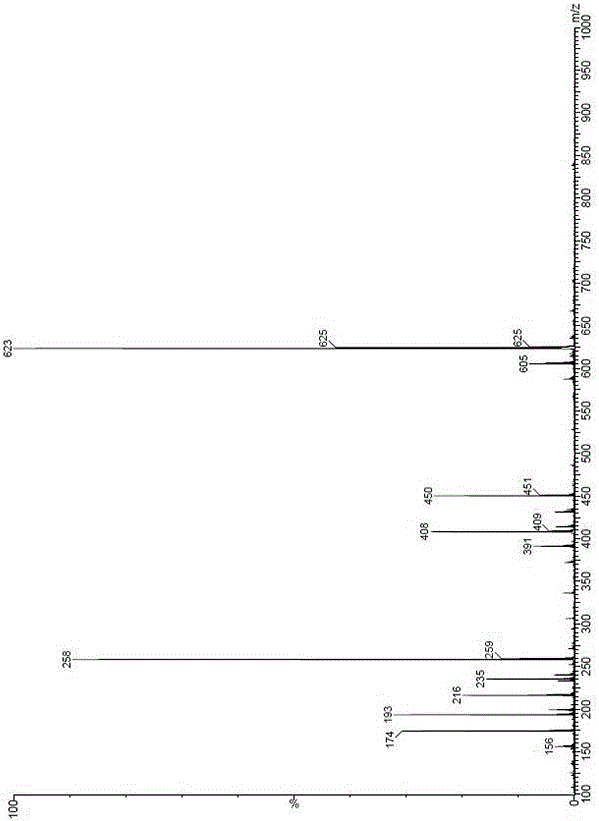
[0065] The preparation of embodiment 3 sitagliptin phosphate impurity C
[0066] 1. Preparation of compound III
[0067] Compound II (20g, 0.06mol), N,N-dicyclohexylcarbodiimide (DCC, 24.74g, 0.12mol), 3-(trifluoromethyl)-5,6,7,8-tetrahydro -[1,2,4]triazolo[4,3,α]pyrazine hydrochloride (SM2, 13.72g, 0.06mol) was added to 300ml N,N-dimethylformamide, and then added at room temperature Triethylamine (18.21g, 0.18mol) and N,N-dimethylaminopyridine (0.73g, 0.006mol) were stirred at room temperature for 24 hours. Filter the reaction system, add 200ml dichloromethane and 50ml water to the filtrate, separate the layers, extract the water layer three times with 100ml dichloromethane, separate the layers, wash the organic layer with saturated sodium bicarbonate and saturated brine respectively, add anhydrous sulfuric acid to the organic layer Dry over magnesium for 1 h, filter, and concentrate the filtrate to dryness to obtain a white solid (ie compound III, 24.36 g, 80%).
[0068] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com