Sitagliptin phosphate tablet and preparation method thereof
A technology of sitagliptin phosphate and disintegrant, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of slow release speed and slow hypoglycemic effect, Achieve the effect of fast dissolution rate, simple preparation process and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
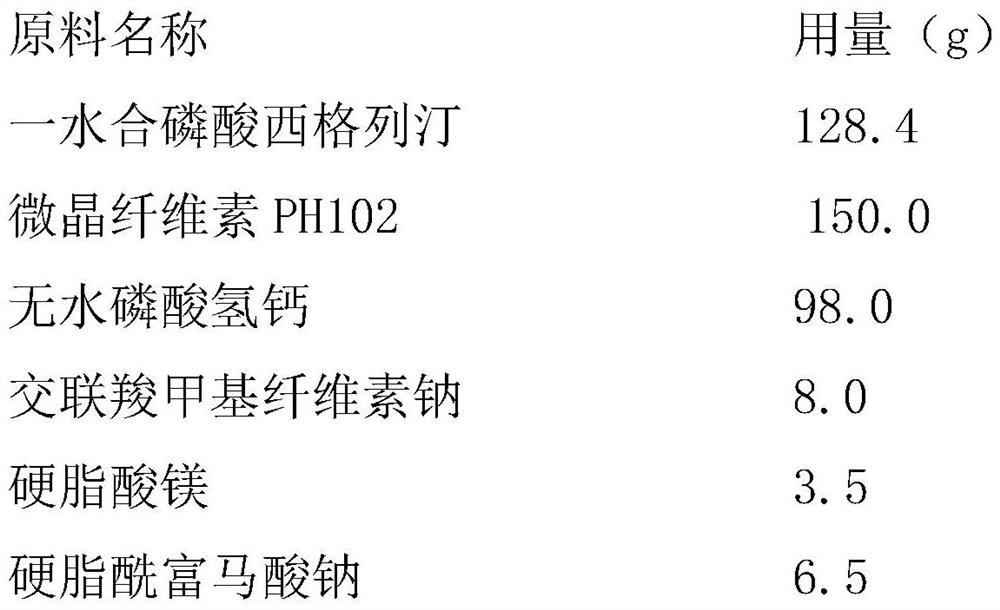
[0029] Example 1: Sitagliptin Phosphate Tablets Prepared by Direct Compression
[0030] The raw material formula (1000 prescription quantities) of sitagliptin phosphate tablet described in the present embodiment is as follows:
[0031]
[0032] The preparation technology of sitagliptin phosphate tablet described in the present embodiment is as follows:
[0033] 1. Screening: Pass sitagliptin phosphate, disintegrant and filler through 80-mesh sieve respectively;
[0034] 2. Premixing: Mix the sieved sitagliptin phosphate, disintegrant and filler evenly to prepare the premixed drug powder;
[0035] 3. Total mixing: add a lubricant to the premixed powder prepared in step 2, and mix evenly to obtain a mixed powder;
[0036] 4. Tablet compression: use a tablet press to compress the mixed medicinal powder prepared in step 3 into a tablet core with a hardness of 8kg±1kg;
[0037] 5. Coating: film-coat the tablet core obtained in step 4, and increase the weight by 3.2%, to obtai...
Embodiment 2
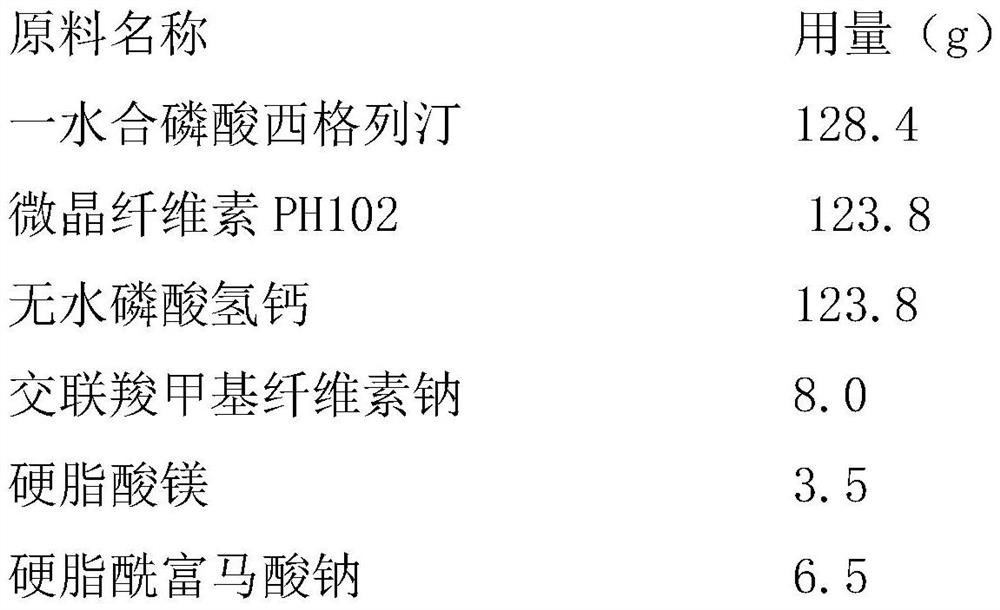
[0038] Embodiment 2: Preparation of Sitagliptin Phosphate Tablets by Direct Compression
[0039] The raw material formula (1000 prescription quantities) of sitagliptin phosphate tablet described in the present embodiment is as follows:
[0040]
[0041] The preparation technology of sitagliptin phosphate tablet described in the present embodiment is as follows:
[0042] 1. Screening: Pass sitagliptin phosphate, disintegrant and filler through 80-mesh sieve respectively;
[0043] 2. Premixing: Mix the sieved sitagliptin phosphate, disintegrant and filler evenly to prepare the premixed drug powder;
[0044] 3. Total mixing: add a lubricant to the premixed powder prepared in step 2, and mix evenly to obtain a mixed powder;
[0045] 4. Tablet compression: use a tablet press to compress the mixed medicinal powder prepared in step 3 into a tablet core with a hardness of 8kg±1kg;
[0046] 5. Coating: film-coat the tablet core obtained in step 4, and increase the weight by 3.5%,...
Embodiment 3
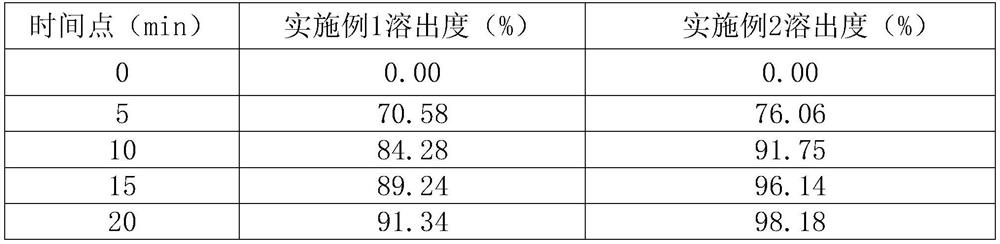
[0052] Using the raw material formula and preparation method of Example 2, the difference is only: when the tablet core is film-coated, the weight gain: 3%, 4.5%, and then two different sitagliptin phosphate tablet products are tested for dissolution rate. Determination, measurement conditions: 900ml water, paddle method, rotating speed 50rpm. The dissolution results are shown in the table below:
[0053] time point (min) Dissolution at 3% weight gain (%) Dissolution at 4.5% weight gain (%) 0 0.00 0.00 5 77.06 82.81 10 91.75 93.75 15 96.14 98.56 20 98.18 102.17 30 100.40 103.04 45 101.89 103.76
[0054] It can be known from the above table that the weight gain of the coating has a certain influence on the dissolution rate of sitagliptin phosphate tablets, and the weight gain should be controlled at 3% to 4.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com