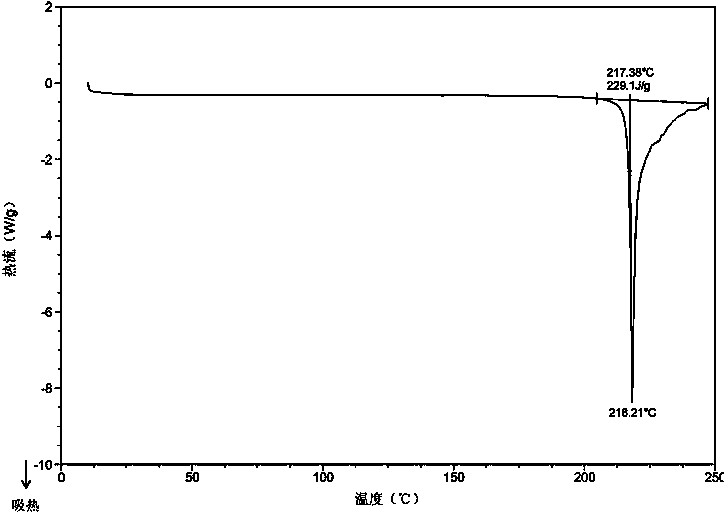
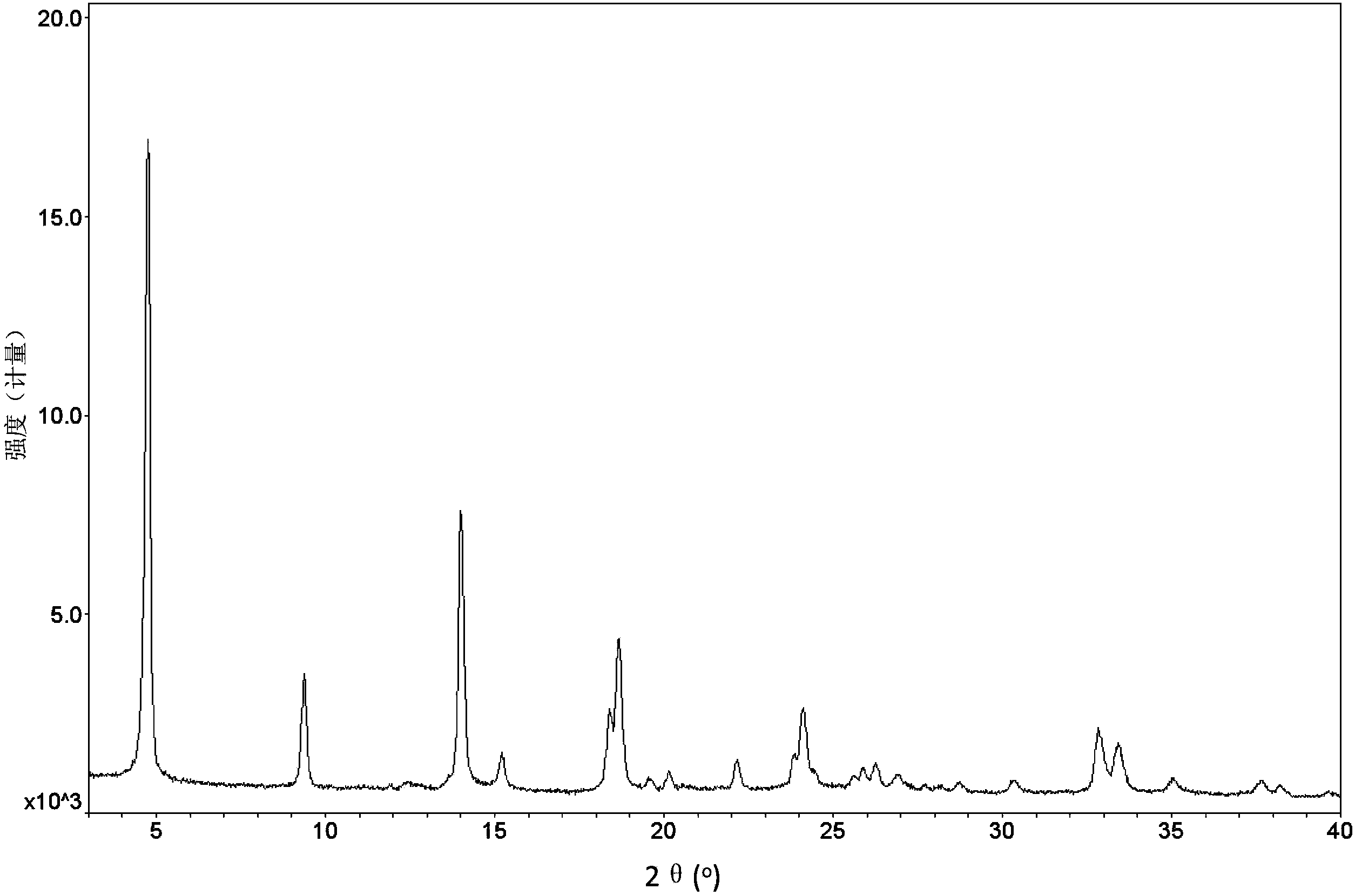
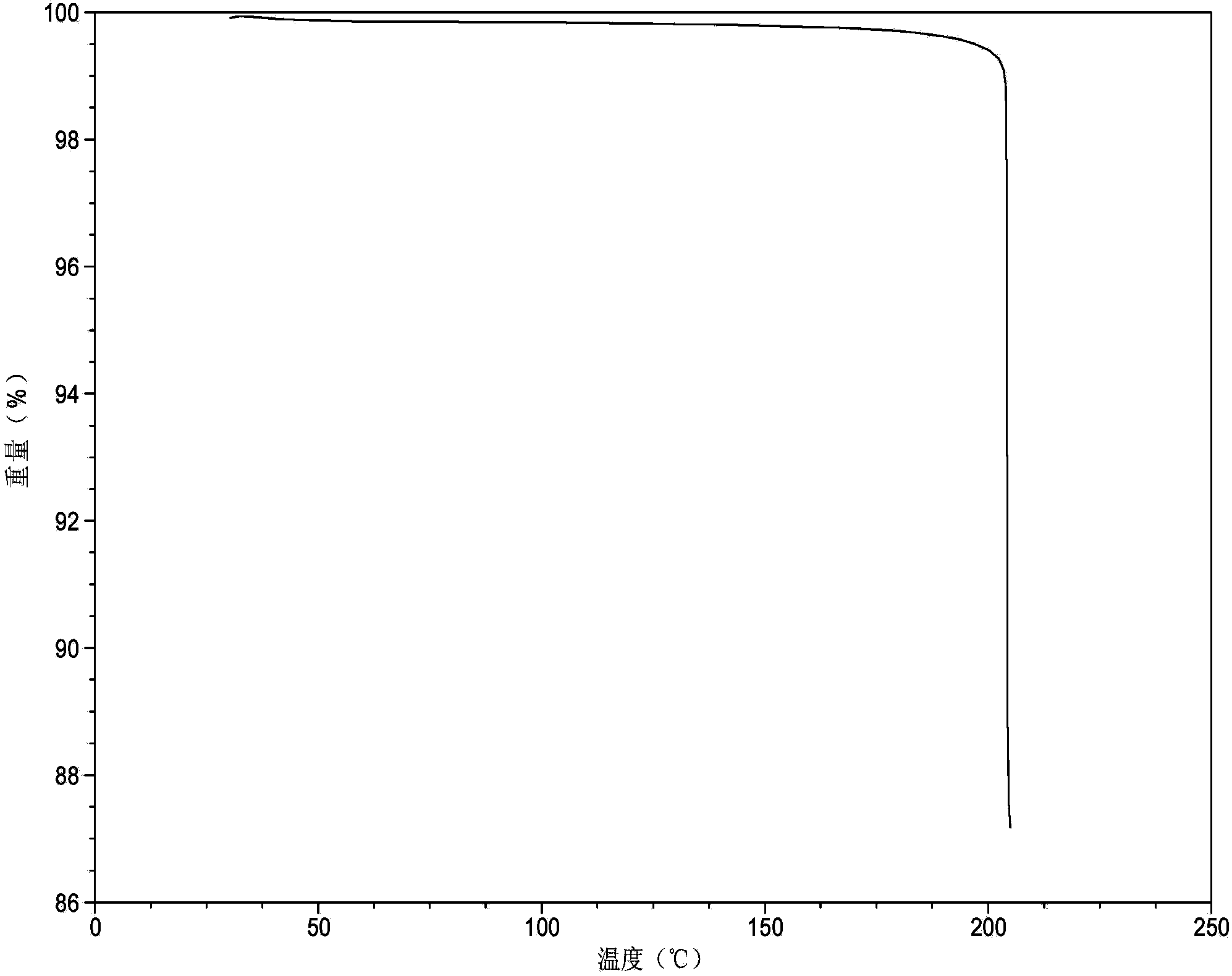
Method for preparing sitagliptin phosphate anhydrous crystal form I
A technology of sitagliptin phosphate and anhydrous crystals, which is applied in the field of pharmaceutical chemical crystallization, can solve problems such as high drying temperature, high crystallization temperature, and complicated process operation, and achieve industrial production, mild reaction conditions, and simple process operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The preparation process of sitagliptin free base is:
[0067]
[0068] Add 20 mL of acetonitrile to a 50 mL round bottom flask, add (3R)-3-[(1,1-dimethylethoxycarbonyl)-amino]-4-(2,4,5-trifluorophenyl) butane acid (3.32g, 0.01mol) and 3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazol[4,3-α]piperazine hydrochloride ( 2.28g, 0.01mol), the temperature of the reaction system was cooled to 0°C with an ice-salt bath, and 1-hydroxybenzotriazole (HOBT) (1.62g, 0.012mol), 1-ethyl-3-(3- Dimethylaminopropyl) carboximide hydrochloride (EDC·HCl) (2.29g, 0.012mol), add 3g of triethylamine dropwise, stir and react at room temperature for 24h, wash the reaction solution with 3×20mL distilled water, and the organic layer Dry over anhydrous magnesium sulfate for 1 hour, filter off the desiccant, and concentrate to obtain 4.81 g of product. 1 H NMR (500MHz, CDCl 3 )δ7.08(dd,J=16.7,8.8Hz,1H),6.98-6.75(m,1H),5.33(d,J=8.6Hz,1H),4.95(s,2H),4.18(s,4H ),3.99(s,1H),2.82(dd,J=128.2,7.2H...
Embodiment 1
[0077] At room temperature, take 1.25g of sitagliptin phosphate anhydrous crystal form IV and add 25mL of acetone to obtain a solid suspension of sitagliptin phosphate, stir the solid suspension at 10°C for 24h, filter the obtained crystal slurry, wash with acetone, and filter the cake Placed in a vacuum oven at 50°C and dried for 6 hours to obtain anhydrous crystal form I of sitagliptin phosphate (yield: 92.0%).
Embodiment 2
[0079] At 40°C, take 1.25g of amorphous sitagliptin phosphate and add 50mL of acetone to obtain a solid suspension of sitagliptin phosphate, then add 62.5mg of sitagliptin phosphate anhydrous form I seed crystals with stirring at 40°C, and stir for 12h , filtered the obtained crystal slurry, washed with acetone, and dried the filter cake in a vacuum oven at 40°C for 2 hours to obtain anhydrous crystal form I of sitagliptin phosphate (yield: 92.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com