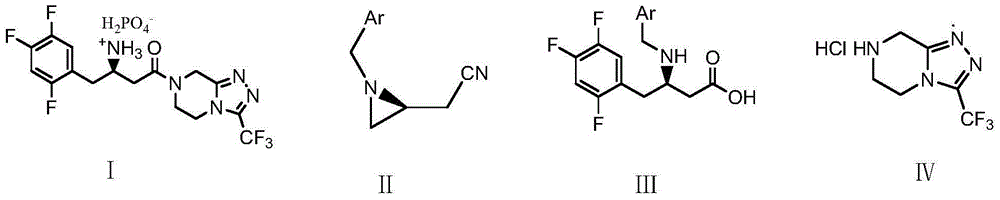
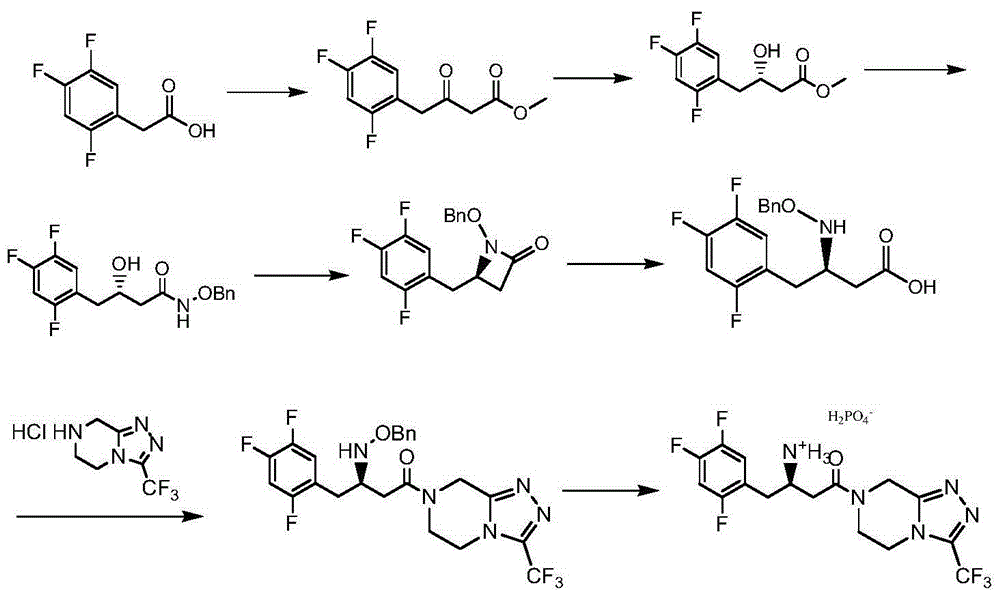
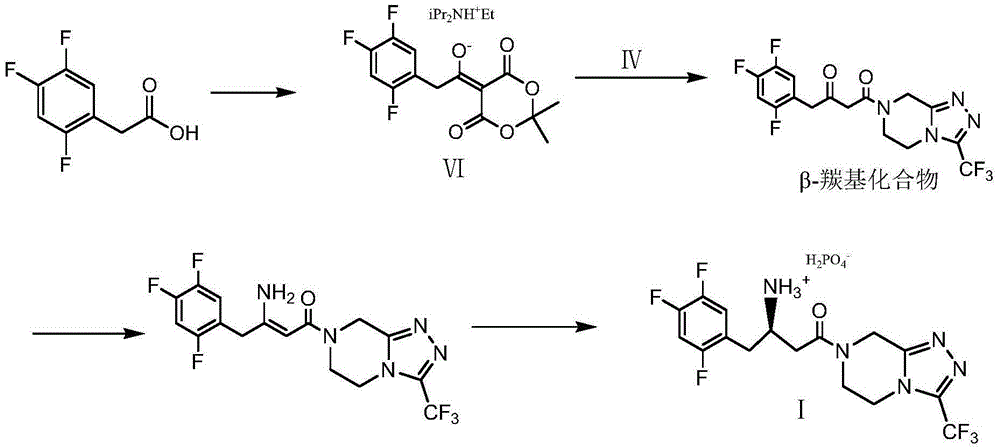
Low-cost preparation method of sitagliptin phosphate
A technology of sitagliptin and phosphate, which is applied in the field of preparation of sitagliptin phosphate, can solve the problem of incapable of using sitagliptin phosphate and the like, and achieves easy operation, simple synthesis process and short process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: the preparation of N-benzyl-2S-cyanomethylacridine (II)
[0075] Add 200 grams of N,N-dimethylformamide, 92.5 grams (1.0 moles) of S-epichlorohydrin, and 107 grams (1.0 moles) of benzylamine to a 500-milliliter four-necked flask, and stir at 10-15°C for reaction 1 Hour. Add 50 grams (1.0 moles) of sodium cyanide powder, stir and react at 20-25°C for 2 hours, filter, add the filtrate dropwise to 262 grams of triphenylphosphine, 202 grams of DIAD (diisopropyl azodicarboxylate) , 300 grams of N,N-dimethylformamide mixture, the temperature of the dropping process is controlled between 10-20°C, and the dropwise addition is completed in 75 minutes. After that, the reaction is stirred at 20-25°C for 8 hours, and the pressure is reduced Distill (50°C, 15mmHg) to recover N,N-dimethylformamide, add 1000 g of water and 300 g of ethyl acetate to the residue, stir for 30 minutes, separate the layers, and extract the aqueous layer three times with ethyl acetate, each ...
Embodiment 2
[0076] Embodiment 2: the preparation of N-benzyl-2S-cyanomethylacridine (Ⅱ)
[0077]Add 200 grams of N,N-dimethylformamide, 92.5 grams (1.0 moles) of R-epichlorohydrin, 50 grams (1.0 moles) of sodium cyanide powder to a 500 milliliter four-neck flask in sequence, The reaction was stirred for 2 hours. 107 g (1.0 mole) of benzylamine was then added, and the reaction was stirred at 15-20° C. for 1 hour. Filter and add the filtrate dropwise to a mixture of 262 grams of triphenylphosphine, 202 grams of DIAD (diisopropyl azodicarboxylate), and 300 grams of N,N-dimethylformamide, and the temperature of the dropping process is controlled at 10 Between -15°C, the dropwise addition was completed in 75 minutes. After that, the reaction was stirred at 20-25°C for 10 hours, and N,N-dimethylformamide was recovered by vacuum distillation (50°C, 15mmHg), and added to the residue 1000 g of water and 300 g of ethyl acetate were stirred for 30 minutes, separated into layers, and the water laye...
Embodiment 3
[0078] Embodiment 3: the preparation of N-benzyl-2S-cyanomethylacridine (Ⅱ)
[0079] 300 g of acetonitrile, 92.5 g (1.0 mole) of S-epichlorohydrin, and 107 g (1.0 mole) of benzylamine were successively added to a 500 ml four-necked flask, and the reaction was stirred at 15-20° C. for 2 hours. Add 50 grams (1.0 moles) of sodium cyanide powder, stir and react at 20-25°C for 2 hours, filter, add the filtrate dropwise to 262 grams of triphenylphosphine, 202 grams of DIAD (diisopropyl azodicarboxylate) , 300 grams of acetonitrile, the temperature of the dropping process is controlled between 15-20°C, and the dropwise addition is completed in 75 minutes. After that, the stirring reaction is between 20-25°C for 8 hours, and the vacuum distillation (50°C, 15mmHg) is recovered Acetonitrile, add 1000 grams of water and 300 grams of ethyl acetate to the residue, stir for 30 minutes, separate layers, extract the water layer with ethyl acetate three times, each time with 100 grams of ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com