Crystalline polymorph of sitagliptin phosphate and its preparation
A technology for sitagliptin phosphate and crystal form, which is applied in the field of sitagliptin phosphate crystal form and its preparation, and can solve problems such as unsuitable industrial production of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Preparation of sitagliptin phosphate crystal form V
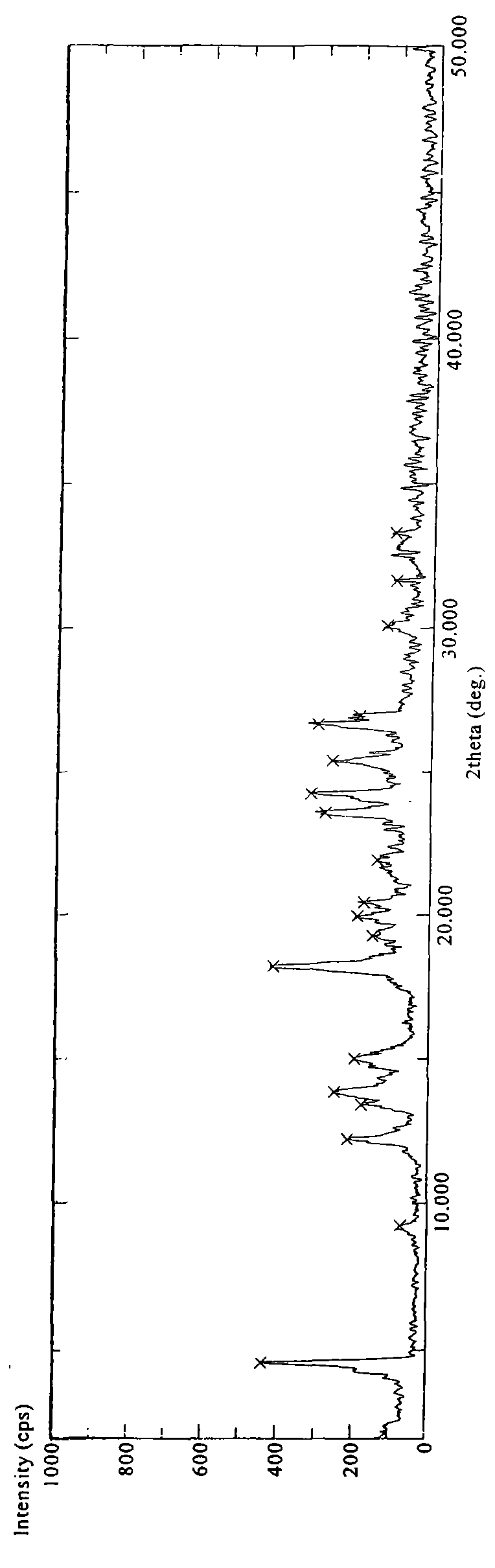
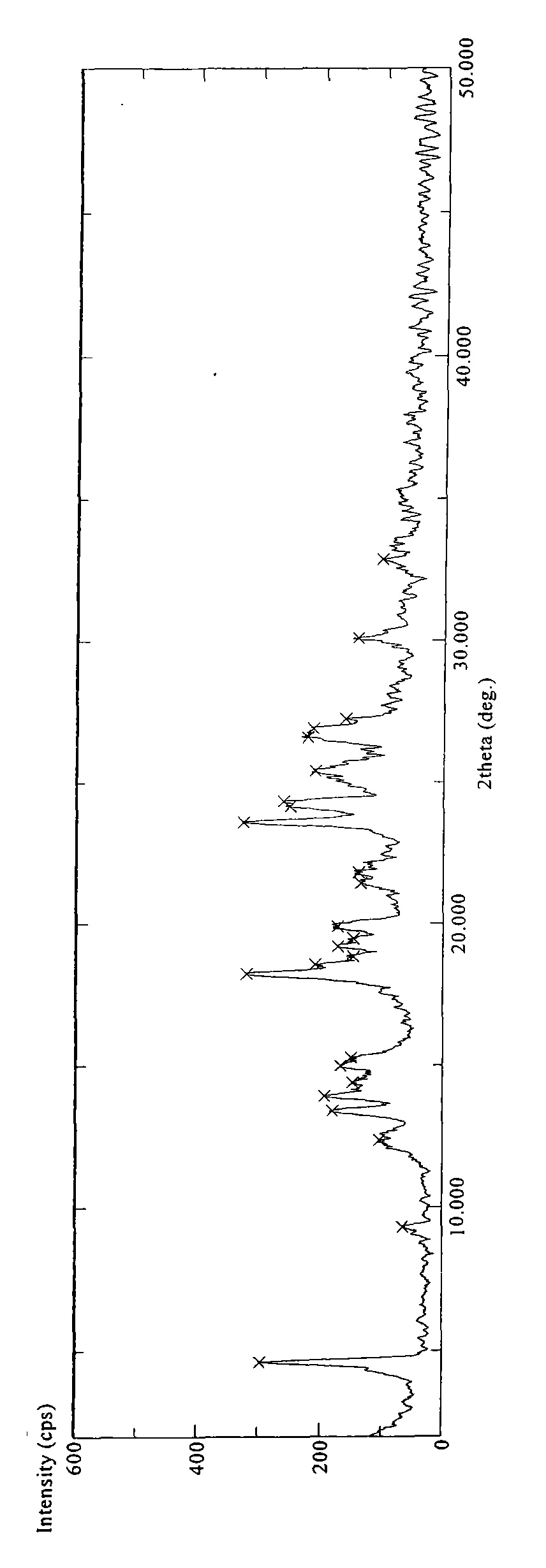
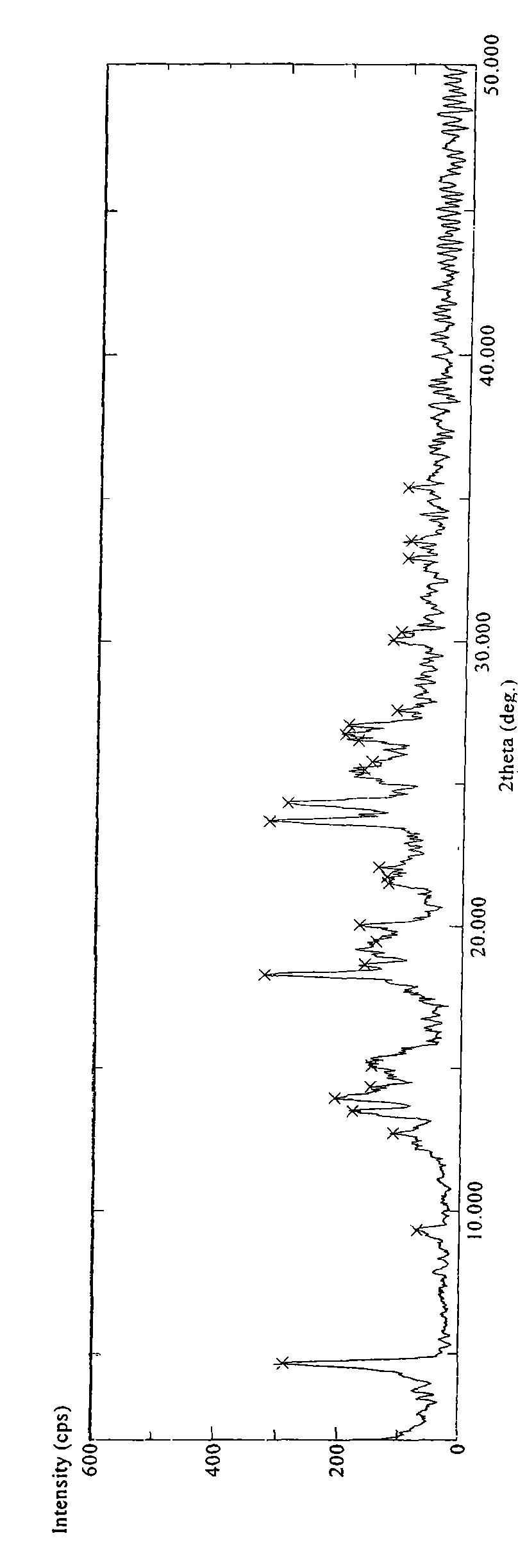
[0084] 4.0 g of sitagliptin phosphate was suspended in 50 ml of heated methanol (HPLC, HPLC grade). Add about 10 ml of water to the suspension and heat until all solid matter is dissolved. The resulting solution was cooled to room temperature and recrystallized overnight at 5°C, no crystals formed and precipitated. Recrystallization was continued at -18°C for three days, and the formed crystals were isolated by filtration and dried in a vacuum oven at about 41°C for 7 hours to obtain a white crystalline substance (about 2.5 g). The obtained product was identified by DSC, TGA and X-ray powder diffraction methods. DSC experiments show that the obtained product has an endothermic peak at about 213.27°C, as Figure 4 shown. The X-ray powder diffraction pattern of gained product is as figure 1 shown. The TGA of the resulting product shows that it contains less than about 0.5% moisture (weight percent), as Figure 7...
Embodiment 2
[0086] Preparation of sitagliptin phosphate crystal form V
[0087] 2.5 g of sitagliptin phosphate was suspended in 5 ml of methanol, 5 ml of n-butanone, 4 ml of tetrahydrofuran, 5 ml of acetonitrile, 5 ml of ethanol and 10 ml of dichloromethane (HPLC, HPLC grade) under heating. Add approximately 7 ml of water to the suspension, heat until all solid matter is dissolved, and mix to form a homogeneous solution. The resulting solution was distilled at about 55°C under reduced pressure (about 20 psi). After most of the organic solvent was distilled off, a small amount of white crystalline material formed on the surface of the flask. The white crystalline substance is placed in an aqueous solution to induce recrystallization of sitagliptin phosphate in the solution, and the suspended solution is cooled to room temperature to continue recrystallization, and a large amount of crystals form and precipitate after 20 to 30 minutes. Recrystallization was continued at room temperature f...
Embodiment 3
[0090] Preparation of sitagliptin phosphate crystal form V
[0091] 4.0 g of sitagliptin phosphate was suspended in 50 ml of heated acetone (HPLC, high performance liquid phase grade). About 7 ml of water was added to the suspension, and the suspension was heated until all solid matter was dissolved and a clear homogeneous solution was obtained. The resulting solution was cooled to room temperature and recrystallized overnight at 5°C, no crystals formed and precipitated. Recrystallization was continued at -18°C for three days, and the formed crystals were isolated by filtration and dried in a vacuum oven at about 41°C for 24 hours to obtain a white crystalline substance (about 2.0 g). The obtained product was identified by DSC and X-ray powder diffraction methods. DSC experiment shows that the obtained product has an endothermic peak at about 211.97°C. The X-ray powder diffraction pattern of gained product is as figure 1 or figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com