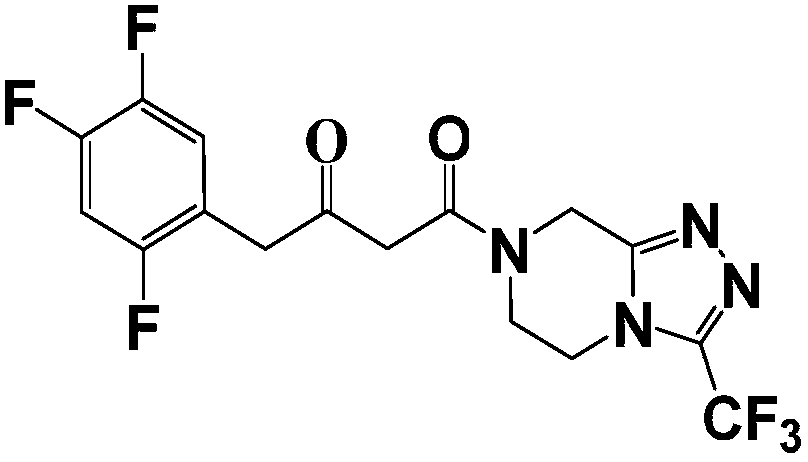
A kind of synthetic method of sitagliptin phosphate intermediate
A technique for the synthesis of sitagliptin phosphate, which is applied in the field of organic synthesis, can solve the problems of difficult recovery of solvent N,N-dimethylacetamide, high cost of pivaloyl chloride, and low product purity, and meet the requirements of reaction equipment Low cost, low cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
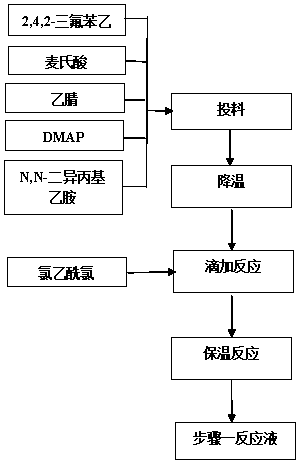
[0029] Step 1. Condensation
[0030] Add 3kg of 2,4,5-trifluorophenylacetic acid, 2.5kg of McBurney's acid, 4.18kg of N,N-diisopropylethylamine, and 0.154kg of DMAP into organic solvent A, protect with nitrogen, and lower the temperature from -10 to 0 After dropping 1.5kg of acetyl chloride, drop it for about 2 hours, then raise the temperature to 0°C for 5 hours, and then raise the temperature to 30-40°C to obtain 5-hydroxy-[(2,4,5-trifluoro The reaction solution of phenyl)-ethylene]-dimethyl-[1,3]dioxo-4,6-dione is directly used in the next step without discharging.
[0031] Step 2, open loop
[0032] Add 3.6 kg of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-α]pyrazine hydrochloride into Step 1 at one time In the reaction solution, after stirring for 0.5 hours at a temperature of 30-40°C, start to add 0.54kg of trifluoroacetic acid dropwise, about 1 hour after the drop, and then raise the temperature to 55-60°C, keep the temperature for 6-8 hours, after the ...
Embodiment 2
[0035] Step 1. Condensation
[0036] With embodiment 1.
[0037] Step 2, open loop
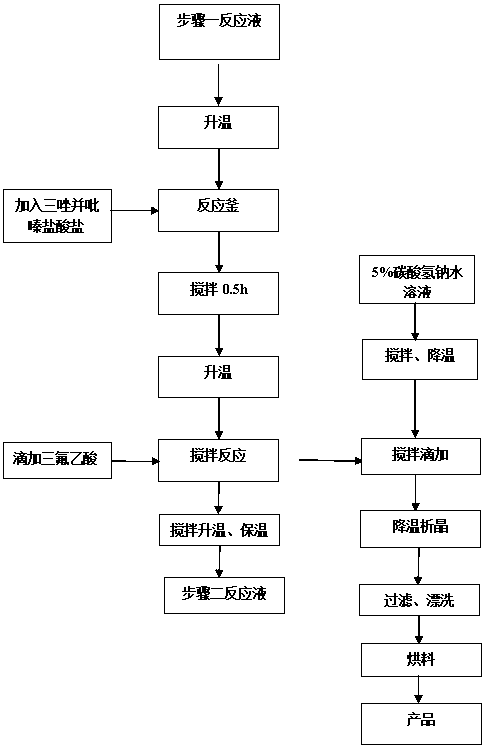
[0038] Add 3.6 kg of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-α]pyrazine hydrochloride into Step 1 at one time In the reaction solution, after stirring for 0.5 hours at a temperature of 30-40°C, start to add 0.54kg of trifluoroacetic acid dropwise, about 1 hour after the drop, and then raise the temperature to 55-60°C, keep the temperature for 6-8 hours, after the end of the heat preservation, Add the reaction solution dropwise to 18L of aqueous solution containing 5% sodium bicarbonate, control the temperature at 10-15°C, and finish dropping in about 3 hours. After suction filtration, and rinse the filter cake with 1.5L of mixed solvent (acetonitrile: water=6:1), the filter cake is sent to a decompression oven for drying, (control temperature 50 ~ 55 ° C, vacuum degree ≥ 0.085MPa) after drying to obtain ( 2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]...
Embodiment 3
[0041] Step 1. Condensation
[0042] With embodiment 1.
[0043] Step 2, open loop
[0044]Add 3.6 kg of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-α]pyrazine hydrochloride into Step 1 at one time In the reaction solution, after stirring for 0.5 hours at a temperature of 30-40°C, start to add 0.54kg of trifluoroacetic acid dropwise, about 1 hour after the drop, and then raise the temperature to 55-60°C, keep the temperature for 6-8 hours, after the end of the heat preservation, Add the reaction solution dropwise to 18L of aqueous solution containing 5% sodium bicarbonate, control the temperature at 10-15°C, and finish dropping in about 3 hours. After suction filtration, and rinse the filter cake with 1.5L of mixed solvent (acetonitrile: water=6:1), the filter cake is sent to a decompression oven for drying, (control temperature 50 ~ 55 ° C, vacuum degree ≥ 0.085MPa) after drying to obtain ( 2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com