Dry granulation process of sitagliptin phosphate composition
A dry granulation technology of sitagliptin phosphate, which is applied to drug combinations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of delayed disintegration time and impurities of sitagliptin phosphate Exceeding the standard, delamination and other problems to achieve the effect of improving stability and disintegration speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
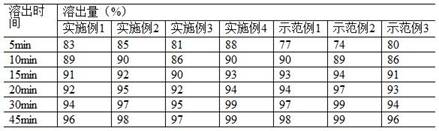
Examples
preparation example Construction
[0017] Wherein the preparation method of tablet, coating is as follows:
[0018] Tabletting: put the granules prepared by this process into the hopper of the tablet press and press them into tablets.
[0019] Coating: Add purified water to the container, slowly add the film coating premix under constant stirring, and stir for at least 45 minutes. Then control the inlet air temperature, main engine speed and inlet air volume, the tablet bed temperature is 42-50°C, the atomization pressure is 0.2-0.4MPa, and the spraying speed is 5-100rpm (depending on the spraying test) for spray coating.
[0020] More preferably, before spraying the liquid, preheat first, and start spraying when the temperature of the tablet bed reaches 42°C. When the weight increases to 2-4%, stop spraying, dry and cool.
[0021] The film-coating premix adopts the common stomach-dissolving film-coating dosage form on the market.
[0022] The API of sitagliptin phosphate has needle-like and rod-like crystal...
Embodiment 1
[0024] 1) Premix, add microcrystalline cellulose, sitagliptin phosphate and anhydrous calcium hydrogen phosphate into the mixing hopper, mix at 15 rpm for 5 minutes, then pass the mixture through a 30-mesh sieve, and then add croscarmellose The plain sodium and sodium stearyl fumarate were passed through a 30-mesh sieve, and then put into the mixing hopper for mixing, and mixed at 15 rpm for 12 min.
[0025] 2) Dry granulation machine, adjust the rotation speed of the pressing roller, the gap of the pressing roller is 0.4-0.5mm, the hydraulic pressure is 3MPa, the crushing speed is 80rpm, the pre-granulation speed is 100rpm, and the final granulation speed is 100rpm. The above and the mixture are dry granulated . The ribbons obtained by granulation are firstly passed through a 2.0mm screen at a suitable speed, and then pulverized by passing through a 1.0mm screen.
[0026] 3) Mixing: Add the dry granulation material into the mixing hopper and mix at 15 rpm for 5 minutes.
[...
Embodiment 2
[0032] 1) Premix, add microcrystalline cellulose, sitagliptin phosphate and anhydrous calcium hydrogen phosphate into the mixing hopper, mix at 15rpm for 10min, then pass the mixture through a 30-mesh sieve, and then add croscarmellose The plain sodium and sodium stearyl fumarate were passed through a 30-mesh sieve, and then put into the mixing hopper for mixing, and mixed at 15 rpm for 15 minutes.
[0033] 2) Dry granulator granulation, adjust the speed of the pressing roller, the gap of the pressing roller is 0.4-0.5mm, the hydraulic pressure is 3.5MPa, the crushing speed is 85rpm, the pre-granulation speed is 105rpm, and the final granulation speed is 100rpm. grain. The ribbons obtained by granulation are firstly passed through a 2.0mm screen at a suitable speed, and then pulverized by passing through a 1.0mm screen.
[0034] 3) Mixing: Add the dry granulated material into the mixing hopper and mix at 15 rpm for 6 minutes.
[0035] Total mixing: manually pass magnesium st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com