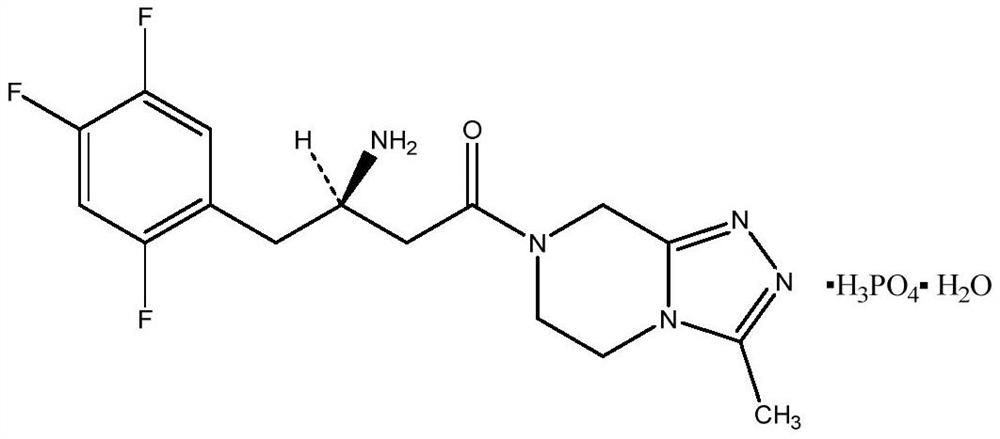
Sitagliptin phosphate tablet and preparation method thereof
A technology of sitagliptin phosphate and tablet cores, which is applied in the field of medicine, can solve the problems of poor fluidity of sitagliptin phosphate, easy sticking, and lower product quality, and achieve low particle size requirements, improve easy sticking, and product quality. high quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
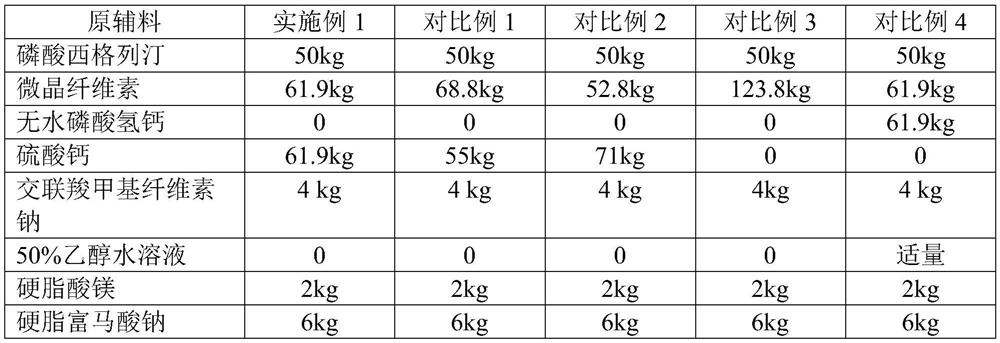
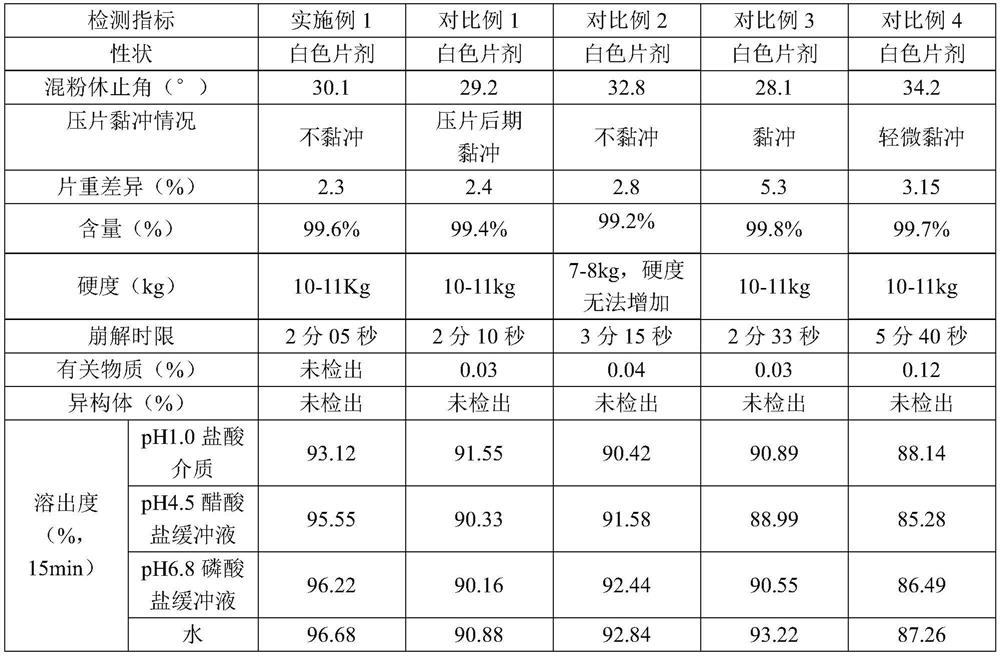
[0032] The preparation methods of embodiment 1, comparative example 1, comparative example 2 and comparative example 3 are as follows:
[0033] (1) Mix sitagliptin phosphate, filler and disintegrant (croscarmellose sodium) for 15min to make it fully mixed; for Example 1, Comparative Example 1 and Comparative Example 2, the filler are microcrystalline cellulose and calcium sulfate; for comparative example 3, the filler is microcrystalline cellulose; for comparative example 4, the filler is microcrystalline cellulose and anhydrous calcium hydrogen phosphate.
[0034] (2) Add lubricant (sodium stearyl fumarate) to the mixture obtained in step (1), mix for 5min, and make it fully mixed evenly.
[0035] (3) Add lubricant (magnesium stearate) to the mixture obtained in step (2), mix for 5min, and make it fully mixed evenly.
[0036] (4) tableting and coating the mixture obtained in step (3), the coating powder used for the coating is Opadry II, the specific model is 85F17438, the c...
Embodiment 2
[0057] For embodiment 2, the lubricant is magnesium stearate, and in the mixture obtained in step (1), magnesium stearate is added, and mixed for 5min to make it evenly mixed;
Embodiment 3
[0058] For embodiment 3, lubricant is calcium stearate, in the mixture obtained in step (1), add calcium stearate, mix 5min, make it mix uniformly;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com