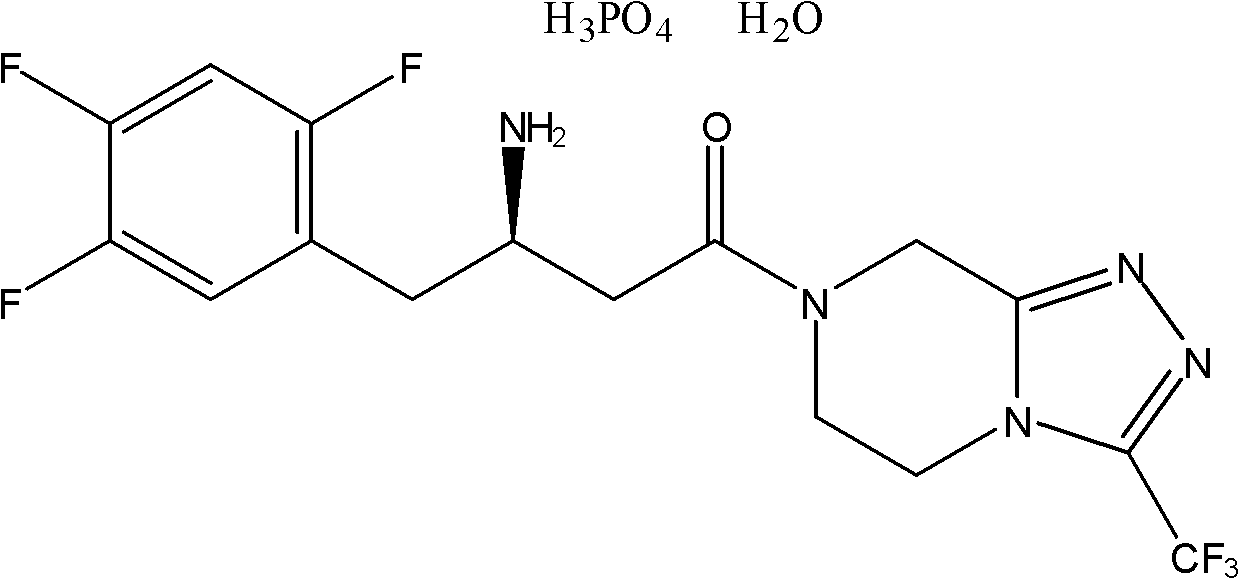
Novel method for synthesizing sitagliptin phosphate and derivative thereof
A technology for sitagliptin phosphate and its derivatives, which is applied in the field of synthesizing sitagliptin phosphate and its derivatives, and can solve problems such as difficulty in increasing the ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
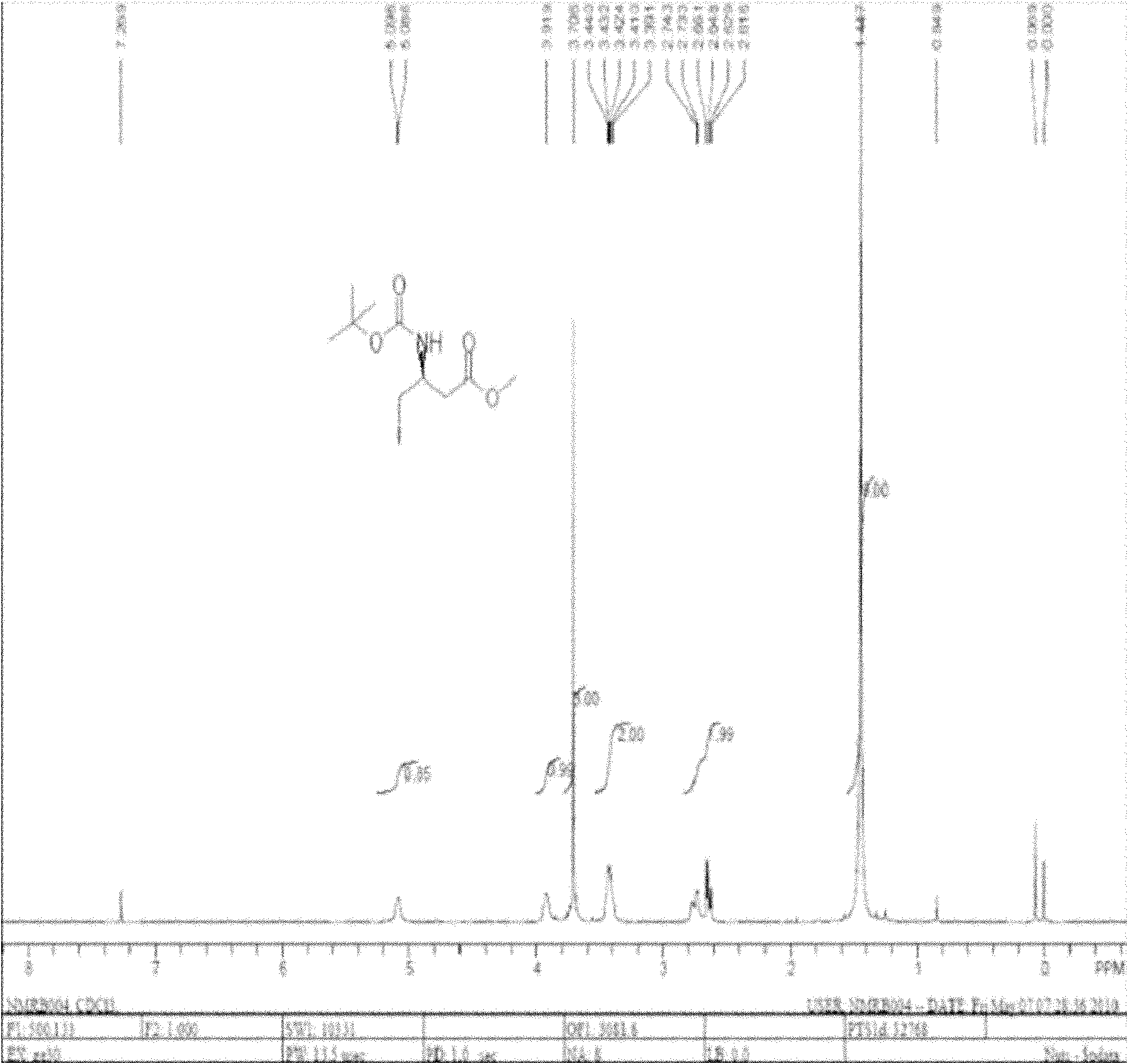
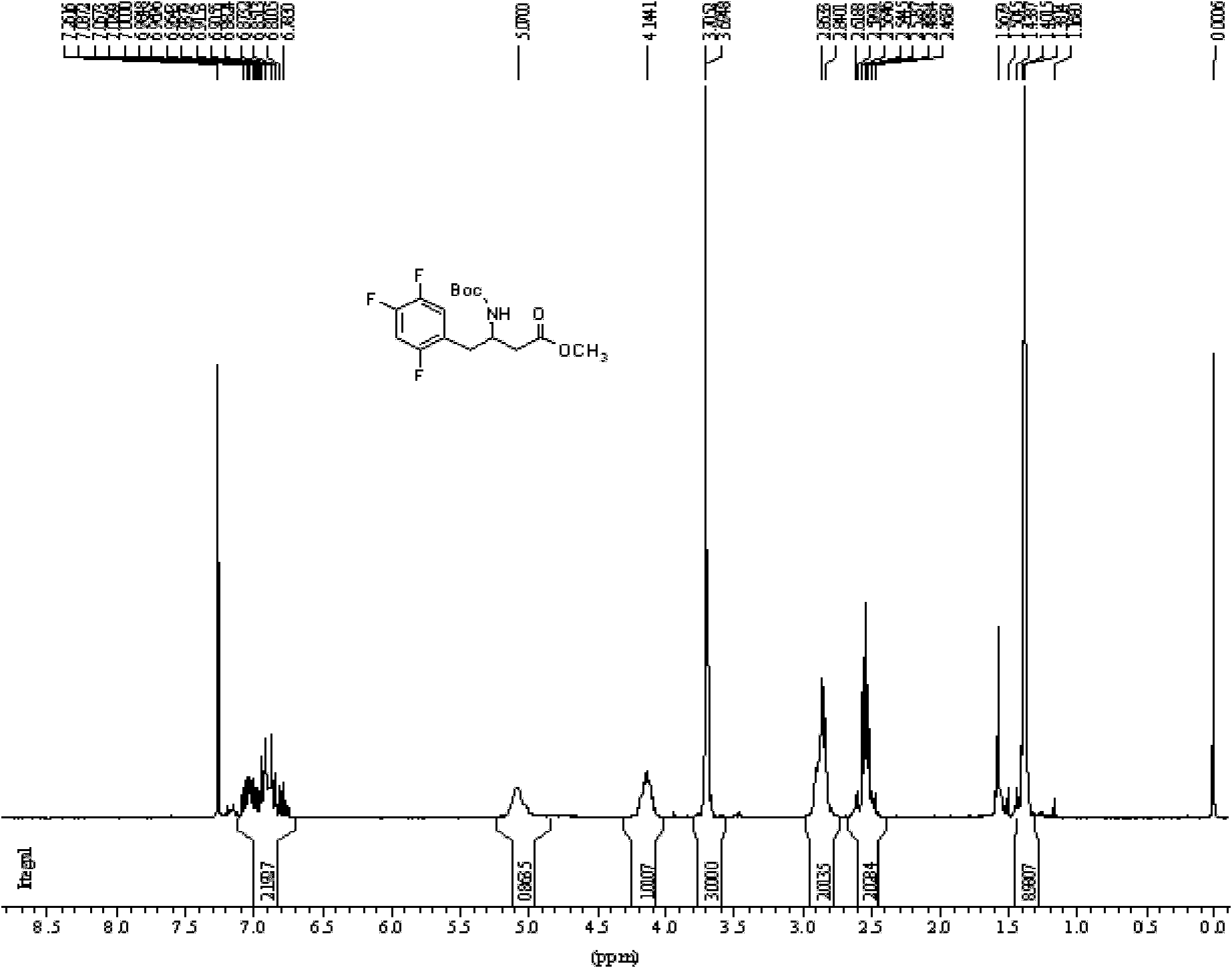
[0051] 1) Amino protection
[0052] Add 20g of L-aspartic acid and 200ml of methanol into a three-necked flask equipped with a magnetic stirrer and a thermometer, add 5ml of thionyl chloride dropwise under cooling, stir at room temperature for 5h after the dropwise addition, concentrate under reduced pressure and remove the solvent to obtain solid 4-L - methyl aspartate hydrochloride 40g;
[0053] In a three-necked flask equipped with a magnetic stirrer and a thermometer, add 20 g of 4-L-aspartic acid methyl ester hydrochloride, 20 g of sodium bicarbonate, dissolve in water and 1,4-dioxane, and dissolve Add BOC anhydride, stir overnight, filter with suction, add 2 L of water to the mother liquor, and adjust the pH with hydrochloric acid. Then extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent to obtain 31 g of light yellow oily liquid N-tert-butoxycarbonyl-4-L-aspartic acid methyl ester;
[005...
Embodiment 2
[0066] 1) amino protection
[0067] Add 22g of L-aspartic acid and 250ml of ethanol to a three-necked flask equipped with a magnetic stirrer and a thermometer, add 12ml of thionyl chloride dropwise under cooling, stir at room temperature for 5 hours after the dropwise addition, concentrate under reduced pressure and remove the solvent to obtain the solid 4-L - ethyl aspartate hydrochloride 40g;
[0068] In a three-necked flask equipped with a magnetic stirrer and a thermometer, add 22 g of 4-L-ethyl aspartic acid hydrochloride, 28 g of sodium bicarbonate, dissolve in water and 1,4-dioxane, and dissolve Add BOC anhydride, stir overnight, filter with suction, add 2 L of water to the mother liquor, and adjust the pH with hydrochloric acid. Then extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure and desolventized to obtain 37 g of light yellow oily liquid N-tert-butoxycarbonyl-4-L-aspartic acid ethyl ester;
[0069] 2) ...
Embodiment 3
[0081] 1) Amino protection
[0082] Add 20g of L-aspartic acid and 200ml of methanol into a three-necked flask equipped with a magnetic stirrer and a thermometer, add 5ml of thionyl chloride dropwise under cooling, stir at room temperature for 5h after the dropwise addition, concentrate under reduced pressure and remove the solvent to obtain solid 4-L - methyl aspartate hydrochloride 40g;
[0083] In a three-necked flask equipped with a magnetic stirrer and a thermometer, add 20 g of 4-L-aspartic acid methyl ester hydrochloride, 20 g of sodium bicarbonate, dissolve in water and 1,4-dioxane, and dissolve Add BOC anhydride, stir overnight, filter with suction, add 2 L of water to the mother liquor, and adjust the pH with hydrochloric acid. Then extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent to obtain 31 g of light yellow oily liquid N-tert-butoxycarbonyl-4-L-aspartic acid methyl ester;
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com