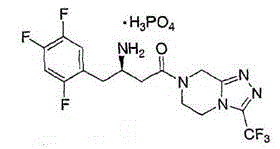
Sitagliptin phosphate composition tablet and preparation method thereof
A technology of sitagliptin phosphate and composition, which is applied in the field of sitagliptin phosphate composition tablet and its preparation, can solve the problems of inconvenient use, slow disintegration speed, low dissolution rate, etc., and achieve easy portability, The effect of fast disintegration speed and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
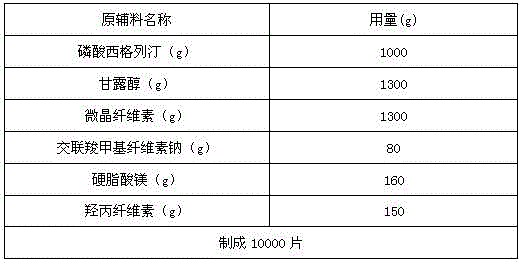
[0030] Production of 1000 specifications is 100mg sitagliptin phosphate composition tablets, the active ingredients of the sitagliptin phosphate composition tablet are sitagliptin phosphate, mannitol, microcrystalline cellulose, cross-linked carboxylate The amounts of sodium methylcellulose, magnesium stearate and 5% aqueous hydroxypropylcellulose solution are shown in Table 1.
[0031]
[0032] Concrete preparation steps are as follows:
[0033] The first step: sieve, pass sitagliptin phosphate through a 100-mesh sieve, and pass mannitol, microcrystalline cellulose and croscarmellose sodium through a 80-mesh sieve;
[0034] The second step: mixing, mixing sitagliptin phosphate, mannitol, microcrystalline cellulose and cross-linked carboxymethyl cellulose sodium after sieving to obtain the first mixture, set aside;
[0035] The third step: granulation, add the weighed 5% hydroxypropyl cellulose aqueous solution to the first mixture to make soft material, pass through a ...
Embodiment 2
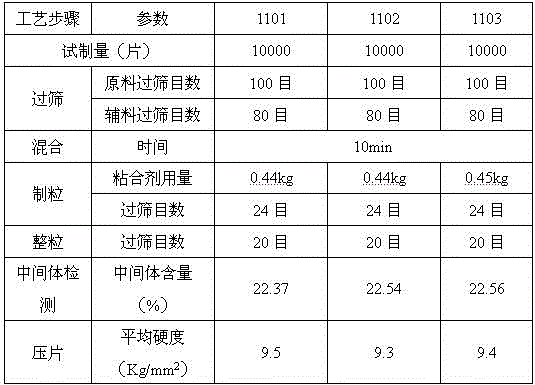
[0042] Adopt the formula of embodiment 1 to carry out scale-up test, the concrete consumption of raw material is as follows:
[0043] Table 3 specification is 100mg sitagliptin phosphate composition tablet scale-up test raw material consumption
[0044]
[0045] The control parameters in the specific process are shown in Table 4:
[0046] Table 4 key process parameter control table
[0047]
[0048] The results show that during the scale-up process, the prescription process of this specification preparation is practical and feasible, and the process is stable. The indexes of the prepared samples were inspected with reference to Example 2, and all the indexes met the quality standards for clinical research.
[0049] The detection instrument and detection method thereof used in above embodiment 1, embodiment 2 technology are summed up as follows:
[0050] 1. Moisture content of granules: Use the Karl Fischer Moisture Analyzer to measure the moisture content of granules ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com