A kind of transaminase and its application in the synthesis of sitagliptin intermediate
A transaminase and transamination technology, applied in the field of bioengineering, can solve the problems of poor stereoselectivity, expensive catalyst, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Cloning of embodiment 1 mycobacterium PYR-1 transaminase gene
[0076] According to the gene sequence predicted to be mycobacterium PYR-1 transaminase included in Genbank (NCBI accession number: YP_955297.1), PCR primers were designed as the upstream and downstream primers for gene cloning in Table 1. Wherein, the underlined part of the upstream primer is the NdeI restriction site, and the underlined part of the downstream primer is the EcoRI restriction site.
[0077] PCR amplification was performed using the genomic DNA of mycobacterium PYR-1 as a template. PCR system: 10×KOD-Plus PCR buffer 2μL, 25mM MgSO 4 1.2 μL, 2 mM dNTP 2 μL, KOD-Plus PCR high-fidelity enzyme 0.3 μL, DNA template 0.5 μL (including 0.1 μg DNA template), ddH 2 013 μL, each 0.5 μL (10 mmol / L) of the gene cloning upstream primers and the gene cloning downstream primers (SEQ ID No: 11 and 12) in Table 1 were used for PCR amplification. PCR amplification steps are: (1) 95°C, pre-denaturation for 3m...
Embodiment 2
[0080] The construction of embodiment 2 recombinant expression vector
[0081] The transaminase gene DNA fragment obtained in Example 1 was double-digested with restriction endonucleases NdeI and EcoRI at 37°C for 8 h, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit. Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET21a digested with NdeI and EcoRI at 16°C overnight to obtain the recombinant expression plasmid pET21a-MvAT.
Embodiment 3
[0082] Embodiment 3 Preparation of recombinant expression transformant
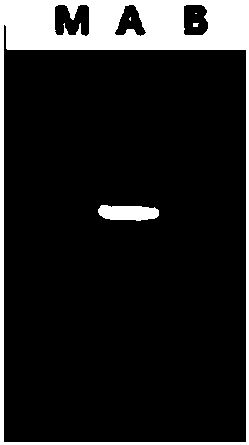
[0083] Transform the recombinant expression plasmid into Escherichia coli (E.coli) DH5α competent cells, the transformation conditions are 45°C, heat shock for 90 seconds, and positive recombinants are screened on the resistance plate containing ampicillin, and picked Monoclonal, positive clones verified by colony PCR (see figure 2 Lane A in ). Cultivate the recombinant bacteria, extract the plasmid after the plasmid is amplified, retransform into E.coli BL21(DE3) competent cells, spread the transformation solution on the LB plate containing ampicillin, and culture it upside down at 37°C overnight to obtain positive recombinant Transformant E.coli BL21(DE3) / pET21a–MvAT, positive clones verified by colony PCR (see figure 2 Lane B in ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com