Sitagliptin phosphate composition, sitagliptin phosphate tablet, and preparation method and application of sitagliptin phosphate composition and sitagliptin phosphate tablet
A technology of sitagliptin phosphate and its composition, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations with non-active ingredients, etc., can solve the problem that the safety and effectiveness of generic drugs are not guaranteed, and it is difficult for the original drug to be developed in vitro. consistency and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
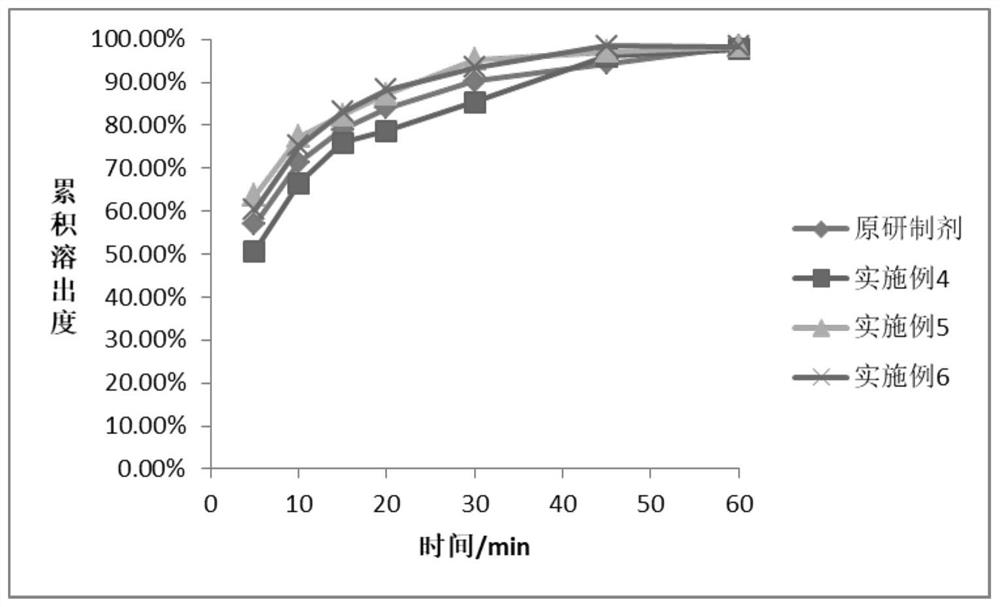
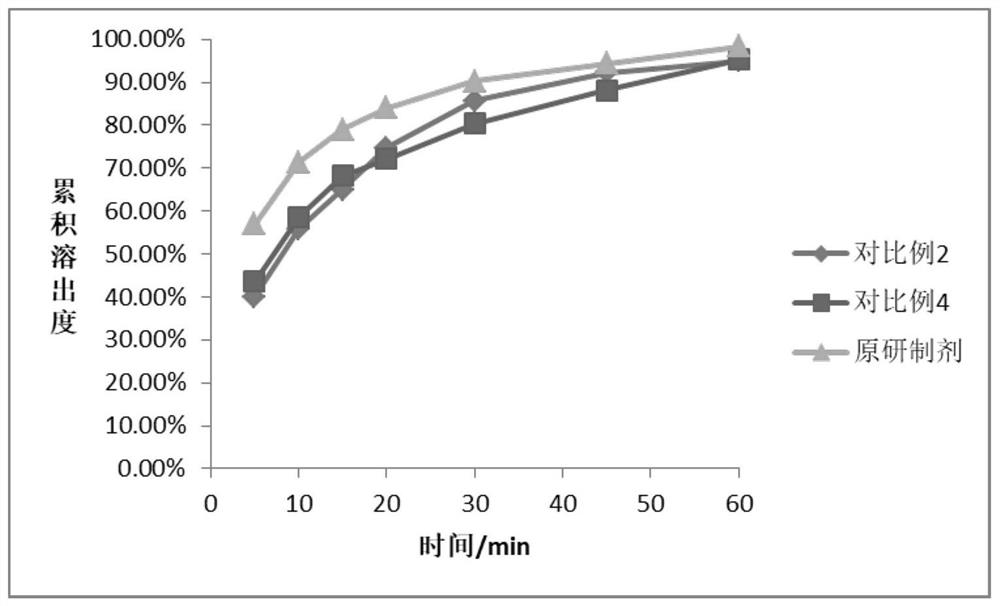
Examples
preparation example Construction
[0066] In the third aspect, the embodiments of the present invention provide a method for preparing the sitagliptin phosphate tablet according to the second aspect, the preparation method comprising the following steps: combining the sitagliptin phosphate tablet described in the first aspect It can be directly compressed into tablets.
[0067] The preparation method of sitagliptin phosphate tablet provided in the embodiment of the present invention adopts powder direct compression technology to reduce granulation, drying and other processes, the preparation process is simple, high in safety, low in cost, saves manpower and material resources, and is more suitable for scale chemical production.
[0068] Further, the preparation method includes the following steps:
[0069] S1. Weigh sitagliptin phosphate monohydrate, calcium hydrogen phosphate anhydrous, microcrystalline cellulose, croscarmellose sodium, magnesium stearate and sodium stearyl fumarate according to the formula q...
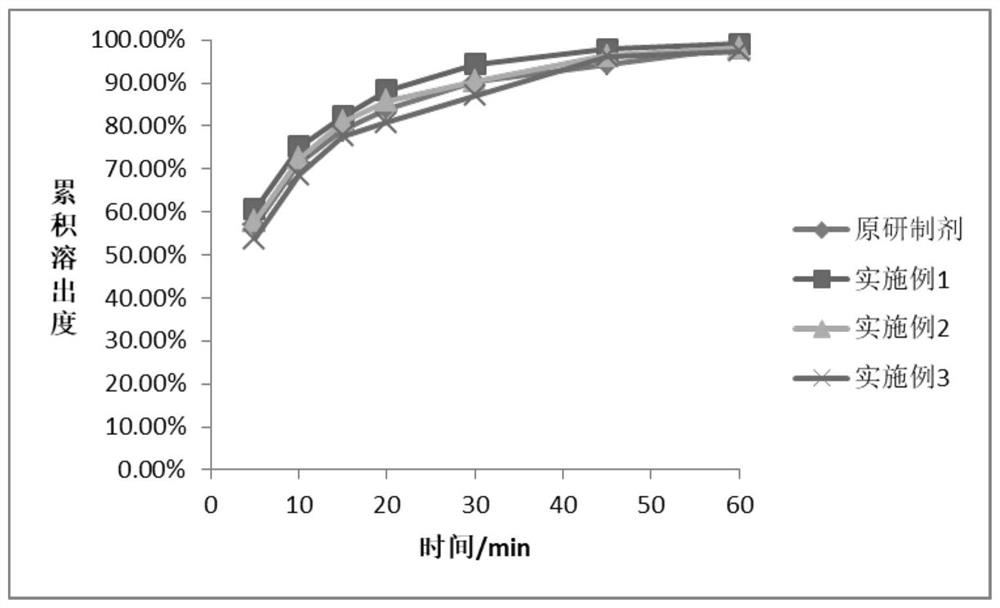
Embodiment 1
[0091] The contents of the components of the sitagliptin phosphate tablets provided in this example are shown in Table 1.
[0092] Preparation:
[0093] S1. Pass anhydrous calcium hydrogen phosphate, croscarmellose sodium, magnesium stearate and sodium stearyl fumarate through a 40-mesh sieve for later use. Weigh sitagliptin phosphate monohydrate (D90 particle size is 30 μm), anhydrous calcium hydrogen phosphate, microcrystalline cellulose (D90 particle size is 260 μm), croscarmellose sodium, hard Magnesium stearate and sodium stearyl fumarate;
[0094] S2. Mix the sitagliptin phosphate monohydrate, anhydrous calcium hydrogen phosphate, microcrystalline cellulose and croscarmellose sodium in a three-dimensional mixer, and the mixing time is 20 minutes to obtain the first mixture;
[0095] S3. Add magnesium stearate and sodium stearyl fumarate to the first mixture, and continue mixing in a three-dimensional mixer for 5 minutes to obtain a second mixture;
[0096] S4. Using a...
Embodiment 2
[0098] The contents of the components of the sitagliptin phosphate tablets provided in this example are shown in Table 1.
[0099] Preparation:
[0100] S1. Pass anhydrous calcium hydrogen phosphate, croscarmellose sodium, magnesium stearate and sodium stearyl fumarate through a 40-mesh sieve for later use. Sequentially weigh sitagliptin phosphate monohydrate (D90 particle size is 40 μm), anhydrous calcium hydrogen phosphate, microcrystalline cellulose (D90 particle size is 260 μm), croscarmellose sodium, hard Magnesium stearate and sodium stearyl fumarate;
[0101] S2~S4 are completely the same as S2~S4 in embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com