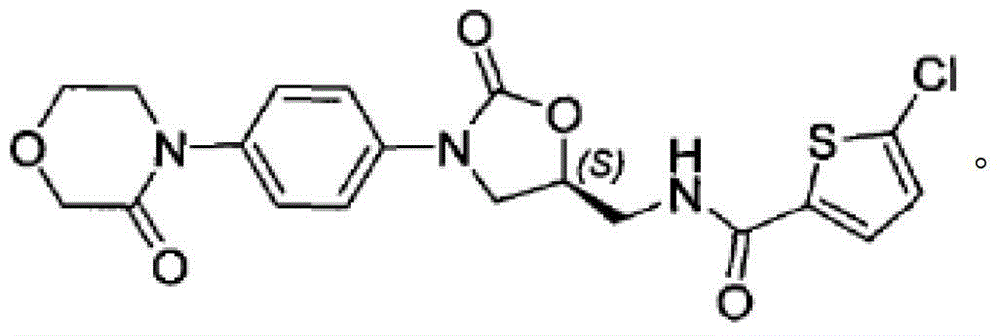
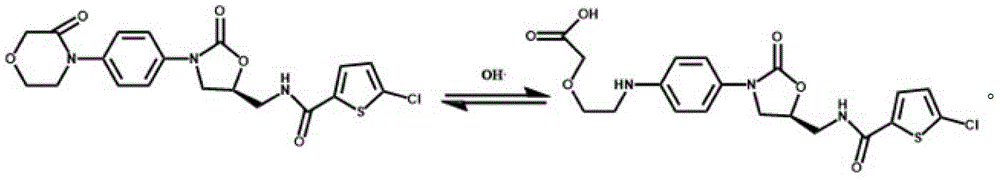
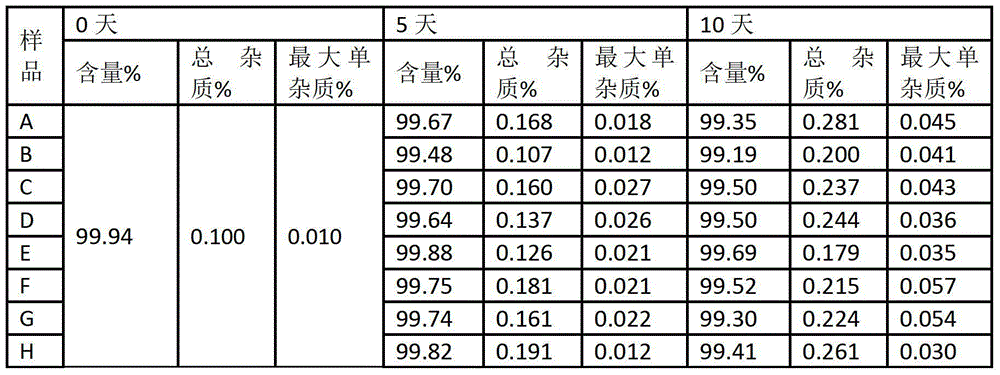
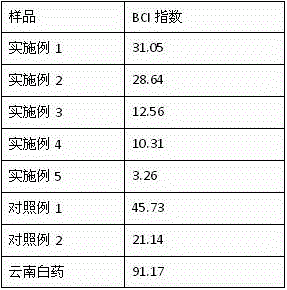
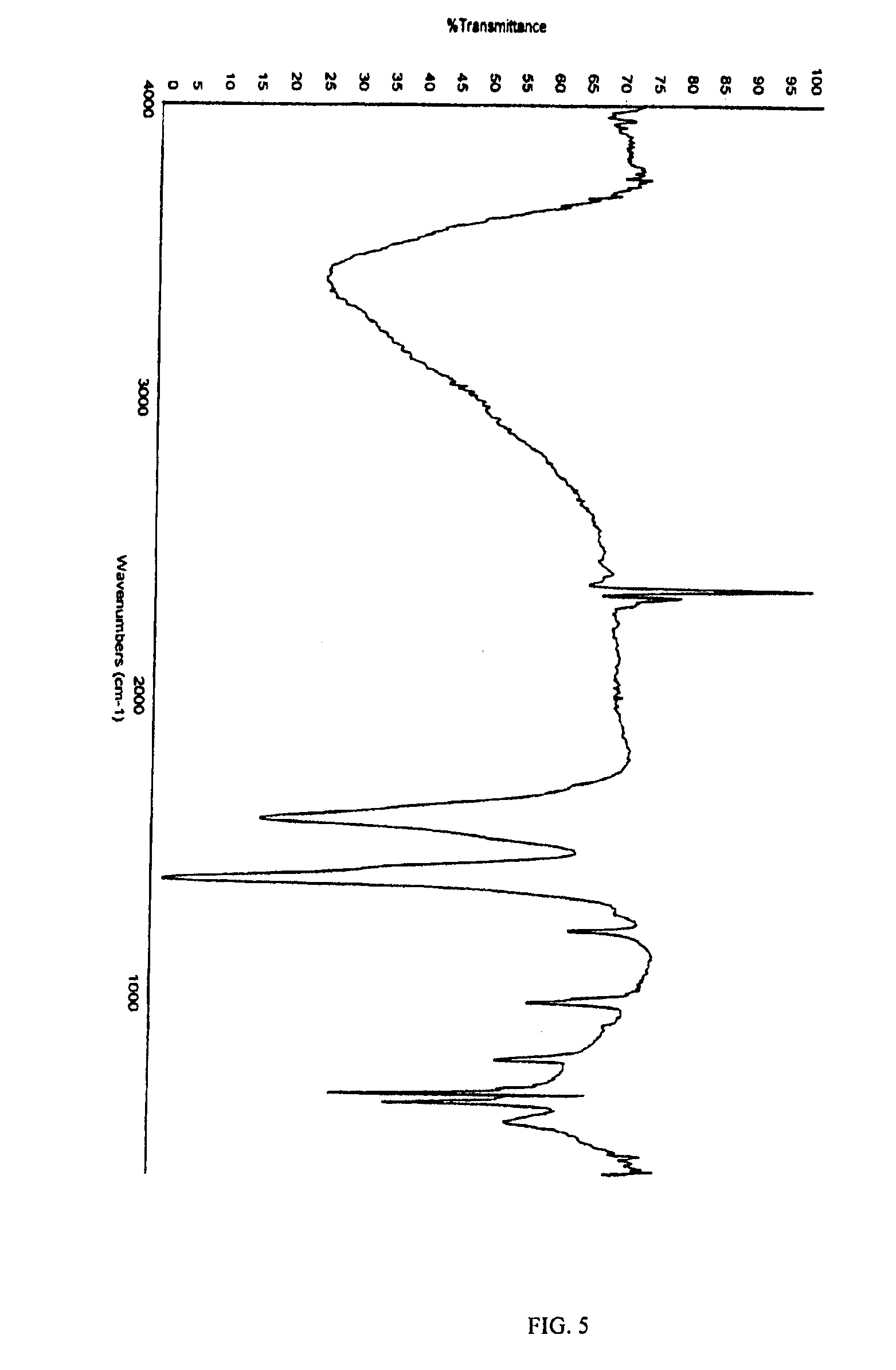
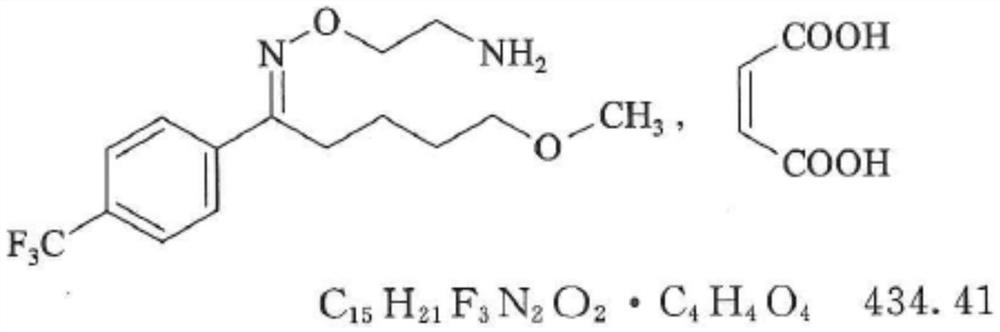
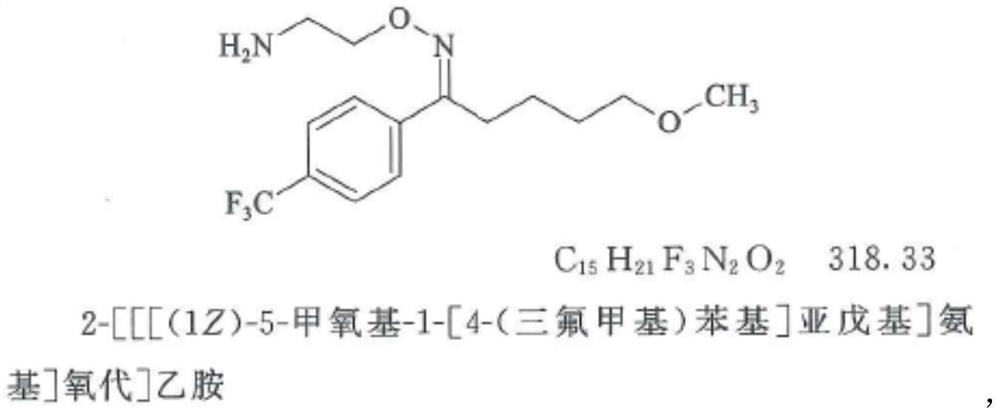
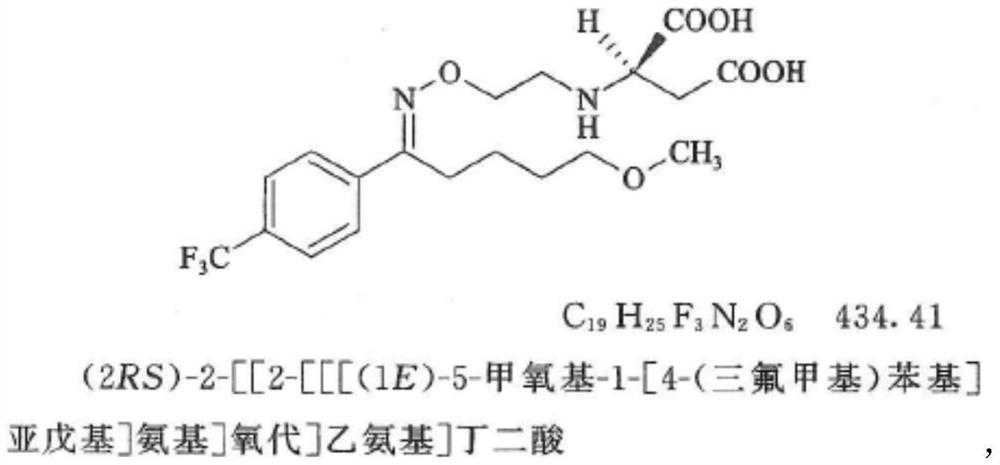
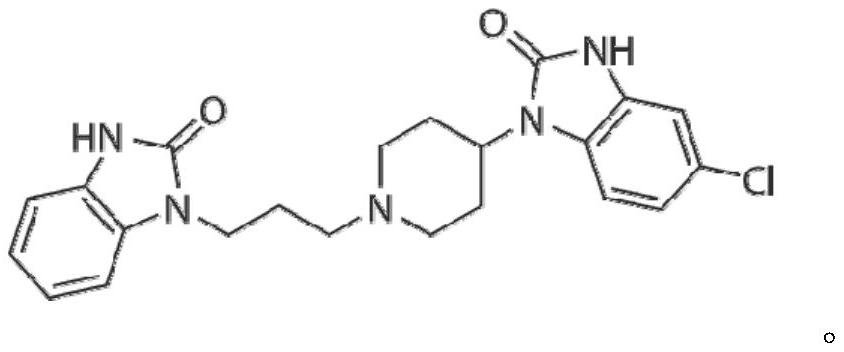
Patents
Literature
78 results about "Sodium fumarate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium fumarate, also called disodium fumarate, is a compound with the molecular formula Na₂C₄H₂O₄. It is the sodium salt of fumaric acid, used as an acidity regulator in processed foods. Sodium fumarate and fumaric acid are sometimes used as terminal electron acceptors in the cultivation of certain anaerobic microorganisms. It appears as an odourless, white, crystalline powder and is soluble in water.
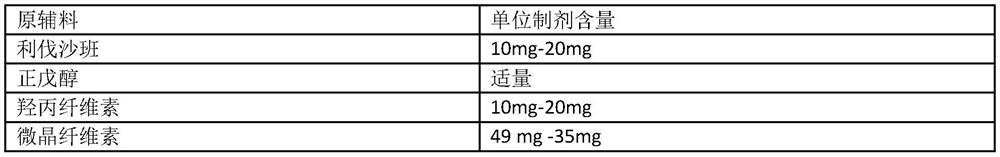
Solid pharmaceutical composition containing rivaroxaban
ActiveCN105232488AGood dissolution effectReduce usageOrganic active ingredientsOrganic chemistryRivaroxabanMedical prescription
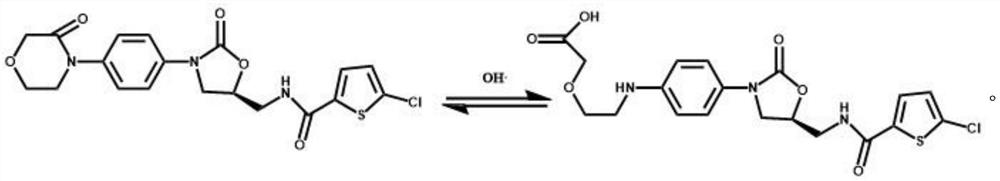
The invention relates to a pharmaceutical composition containing rivaroxaban, in particular to a solid pharmaceutical composition containing rivaroxaban and a preparation method of the solid pharmaceutical composition. The solid pharmaceutical composition containing the rivaroxaban can be further prepared into a film-coated tablet by a specific preparation technology to serve as a specific administration mode. The solid pharmaceutical composition containing the rivaroxaban and the preparation method of the solid pharmaceutical composition have the advantages that by application of the specific preparation technology, using surfactants in a preparation is avoided and dissolution rate of the tablet is increased effectively; by means of using sodium stearyl fumarate in the composition, marked increase of degraded impurities I during long-term storage of the tablet is avoided effectively; through stability accelerating research, the film-coated tablet containing the rivaroxaban, prepared by the prescription and technological steps, is stable and controllable in quality.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Kudzuvine root drink capable of relieving or neutralizing the effect of alcohol and production technique thereof
The invention discloses a Kudzuvine root drink capable of relieving or neutralizing the effect of alcohol and its preparation, wherein the drink comprises (by weight parts) root of kudzu vine extract 600-800, water 200-300, cane sugar 40-60, glucose 140-160, L-ascorbic acid 0.1-0.3, sodium succinate 0.3-0.5, sodium fumarate 0.3-0.5, L-malic acid0.3-0.5, L-tartaric acid 0.3-0.5, lactic acid 0.1-0.2, acetic acid 0.1-0.2, beta cyclodextrin 0.4-0.6, L-leucine 0.45-0.6, L-sulfo-aminolactic acid 0.45-0.6, sodium glutamate 0.4-0.6, L-alanine 0.4-0.6, glycine 0.5-0.6, catechin 0.1-0.3.
Owner:益阳市世纪塑业科技有限公司
Film coating
A film coating composition suitable for use in coating pharmaceutical formulations comprising a) an acrylic polymer dispersion, e.g. an ethylacrylate / methylmethacrylate copolymer such as Eudragit NE30D, b) a surfactant, c) sodium stearyl fumarate, and d) a water-containing liquid useful for the achievement of controlled release from pharmaceutical formulations such as tablets, pellets, etc.
Owner:ASTRAZENECA AB
Method for drying biological cellulose hydrogel
ActiveCN102875847AImprove mechanical propertiesReduce chippingDrying using combination processesDrying solid materials without heatCelluloseThermal insulation
The invention relates to a method for drying biological cellulose hydrogel. The method comprises the following steps of: soaking part of dehydrated biological cellulose hydrogel in a surfactant-containing solution, and then drying. The surfactant is one or more of glycerin fatty acid ester, sucrose fatty acid ester, soyabean lecithin, acetin, tartaric acid glyceride, diacetyl tartaric acid glyceride, citrate, polyglycerol fatty acid ester, stearoyl citrate, stearyl tartrate, sodium stearyl lactate, calcium stearyl lactate, sodium stearyl fumarate and sorbitan fatty acid ester. By the method for drying the biological cellulose hydrogel, the damage to the spatial net structure of biological cellulose in the drying process can be reduced, and the cracking in the drying, packaging and conveying processes is reduced, and the thermal insulation property of a biological cellulose dried product can be improved.
Owner:HAINAN GUANGYU BIOTECH
Chitosan-based hemostatic material and preparation method thereof
InactiveCN105617451AGood anti-adhesion effectPromote repairSurgical adhesivesPharmaceutical delivery mechanismBiocompatibility TestingGlycerol
The invention discloses a chitosan-based hemostatic material and a preparation method thereof. The chitosan-based hemostatic material is prepared from, by mass, 20-40 parts of chitosan, 8-12 parts of corn starch, 1-5 parts of whey protein, 2-5 parts of wheat starch, 1-5 parts of glucose, 2-5 parts of sodium alginate, 2-10 parts of pectin, 1-4 parts of lecithin, 1-4 parts of citric acid, 1-3 parts of sodium fumarate, 1-4 parts of sodium chloride and 8-15 parts of polyhydric alcohol, wherein polyhydric alcohol adopts ethanol or glycerol, and preferably, 2-6 parts of vitamin C and 1-3 parts of vitamin E are included. The chitosan-based hemostatic material has the advantages that the excellent anti-bacterial, hemostatic and analgesic effects are achieved, defective tissue repairing can be facilitated, the anti-adhesive effect is good, the biocompatibility is achieved, the safety are good, the antigenicity does not exist, use is safe, the preparation method is simple, and use is convenient.
Owner:SUZHOU BEC BIOLOGICAL TECH
Method for controlling harmful microbe in preparation process of quick frozen vegetables food
InactiveCN1969692AStrong seasoning bufferDoes not affect antibacterial and antibacterial abilityFruits/vegetable preservation by heatingFruits/vegetable preservation by freezing/coolingMicroorganismSodium fumarate
The invention discloses a controlling method of harmful microbe in the food manufacturing course of rapid-freezing opsonic food, which comprises the following steps: blending inhibitor in the compound of vegetable and water with the weight rate of compound and inhibitor at 100: 1-4 evenly; sterilizing vegetable at 98 deg.c; cooling in the vacuum; freezing; allocating inhibitor with 55%-67% dehydrosodium acetate, 19%-37% sodium gly acid and 3%-17% sodium fumarate.
Owner:NINGBO JIAYI FOOD
Novel environment-friendly alkali-resisting dye penetrant
The invention discloses a novel environment-friendly alkali-resistant dyeing penetrating agent, which comprises the following components in parts by weight: 5-10 parts of dithiocyanomethane, 3-6 parts of fatty alcohol succinate, polyvinyl benzyl trimethyl quaternary 2‑4 parts of ammonium salt, 15‑20 parts of secondary alkyl sodium sulfonate, 3‑7 parts of didecyldimethylammonium chloride, 5‑6 parts of sodium fumarate, 4‑8 parts of vinyl acetate, sulfonated Dioctyl succinate sodium salt 3-7 parts, acrylic acid 2-6 parts, sodium citrate 4-8 parts, sucrose ester 12-16 parts, potassium oxalate 2-6 parts. The environmental-friendly alkali-resistant dyeing penetrant of the present invention has excellent leveling performance, can significantly increase the absorption rate of dyes, is safe and harmless, and is environmentally friendly; it also has excellent strong alkalinity, and the raw materials required for the preparation of the penetrant are cheap and easy to obtain , the preparation process is simple, and the requirements for production equipment are low.
Owner:刘慧宝
Anti-caking compound carbaspirin calcium powder and preparation method thereof
InactiveCN108606953ASimple ingredientsImprove securityOrganic active ingredientsPowder deliveryAnticaking agentPolyethylene glycol
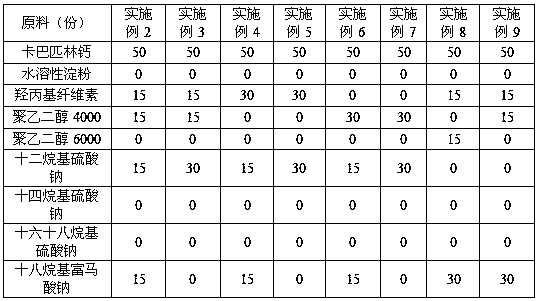
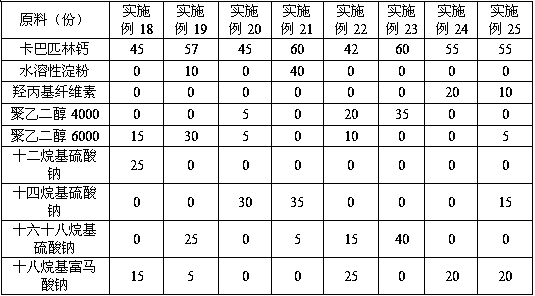
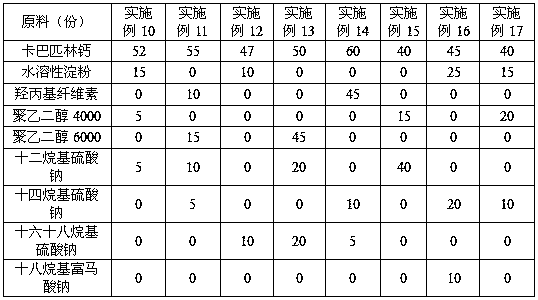
The invention discloses anti-caking compound carbaspirin calcium powder which is prepared from the following raw materials in parts by weight: 40-60 parts of carbaspirin calcium, 10-45 parts of vectorand 5-40 parts of anti-caking agent; the vector is one or a combination of two of water-soluble starch, hydroxy propyl cellulose, polyethylene glycol 4,000 and polyethylene glycol 6,000; and the anti-caking agent is one or a combination of two of lauryl sodium sulfate, sodium tetradecyl sulfate, sodium cetostearylsulfate and sodium octadecyl fumarate. As for the anti-caking compound carbaspirin calcium powder provided by the invention, the components are simple, raw materials are all commercially available raw materials, are cheap and available and are high in safety, so that the caking phenomenon of carbaspirin calcium is effectively inhibited, the storage performance and the use performance of the carbaspirin calcium are improved, the drug property stability of the carbaspirin calcium is ensured at the same time, and the anti-caking compound carbaspirin calcium powder has great practical value; and in addition, a preparation technology is simple, and simple mixing is needed only.
Owner:ZHENGZHOU HOUYI PHARMA
Metal etching agent used for etching copper-containing metal layer and preparation method for metal etching agent
InactiveCN105386056AIncrease etch rateImprove etching efficiencySemiconductor/solid-state device manufacturingDecompositionSodium fumarate
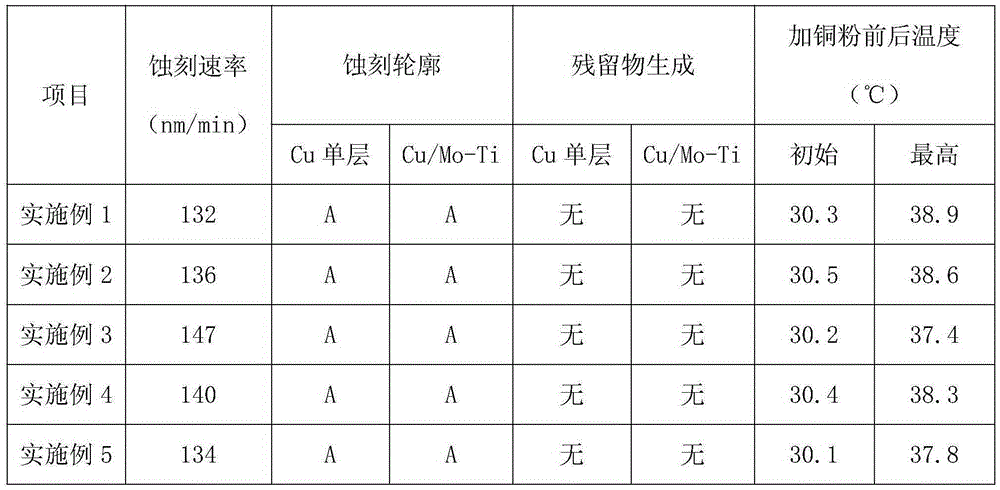
The invention discloses a metal etching agent used for etching a copper-containing metal layer and a preparation method for the metal etching agent. The metal etching agent comprises, by weight, 20-30 parts of hydrogen peroxide, 1-5 parts of acetic acid, 1-3 parts of nitrilotriacetic acid, 10-15 parts of sodium carboxymethyl cellulose, 15-20 parts of boric acid, 12-14 parts of sodium fumarate, 1-2 parts of sodium fluoride, 4-10 parts of ethylene glycol, 2-10 parts of benzidine yellow, 6-8 parts of barium stearate and 8-12 parts of azodiisobutyronitrile. The invention further provides the preparation method for the metal etching agent used for etching the copper-containing metal layer. The etching speed of the metal etching agent is above 132 nm / min, and is far higher than that of common etching liquid, so that the etching efficiency is improved. Under the condition of adding copper powder, the metal etching agent only has the highest temperature of 38.9 DEG C, and therefore the decomposition of the effective component hydrogen peroxide is restrained, and the stability is obviously improved.
Owner:NINGBO DONGSHENG INTEGRATED CIRCUIT ELEMENT
Method for promoting accumulation of astaxanthin in haematococcus pluvialis
ActiveCN113512575AIncrease contentReduce dosageUnicellular algaeClimate change adaptationBiotechnologyHaematococcus
The invention discloses a method for promoting accumulation of astaxanthin in haematococcus pluvialis. The method comprises the following steps: culturing haematococcus pluvialis cells to reach a platform phase, centrifuging the haematococcus pluvialis cells, inoculating the centrifuged haematococcus pluvialis cells into a new culture medium to form a haematococcus pluvialis solution, and adding sodium fumarate into the haematococcus pluvialis solution during light induction to promote accumulation of astaxanthin. According to the method disclosed by the invention, astaxanthin accumulation in haematococcus pluvialis cells can be remarkably accelerated, the content of astaxanthin in haematococcus pluvialis is increased, the yield of astaxanthin produced by large-scale culture of haematococcus pluvialis is increased, and comprehensive economic benefits are improved.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Compound polysaccharide anti-hemorrhagic thin film and preparation method thereof
InactiveCN105770970AGood hemostasisRapid hemostasisPharmaceutical delivery mechanismAbsorbent padsBleeding timeSodium fumarate
The invention discloses a compound polysaccharide anti-hemorrhagic thin film and a preparation method thereof.The thin film is prepared from, by mass, 10-20 parts of chitosan, 2-8 parts of glucose, 3-6 parts of methylcellulose, 1-5 parts of gelatin, 4-7 parts of pectin, 4-7 parts of sodium trimetaphosphate, 8-20 parts of polyhydric alcohols, 5-8 parts of agar, 2-8 parts of sorbic acid, 5-10 parts of sodium alginate, 3-6 parts of panax notoginseng, 1-3 parts of sodium fumarate and 2-5 parts of sanguisorba officinalis.A polysaccharide serves as a matrix, proper anti-hemorrhagic components and auxiliaries are added, and the film-shaped anti-hemorrhagic material is prepared.Compared with conventional powder or spray, the film-shaped anti-hemorrhagic material can stop bleeding in two aspects synchronously.In the early time of bleeding, the film body is similar to tourniquet and can quickly stop bleeding, the anti-hemorrhagic components in the film synchronously stop bleeding at the same time, and the thin film has dual anti-hemorrhagic functions, greatly shortens bleeding time and is obvious in anti-hemorrhagic effect and convenient to use.
Owner:SUZHOU BEC BIOLOGICAL TECH
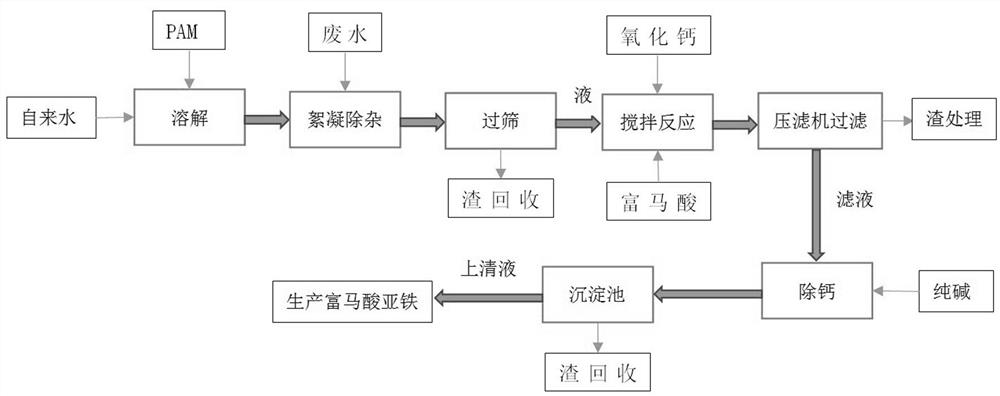
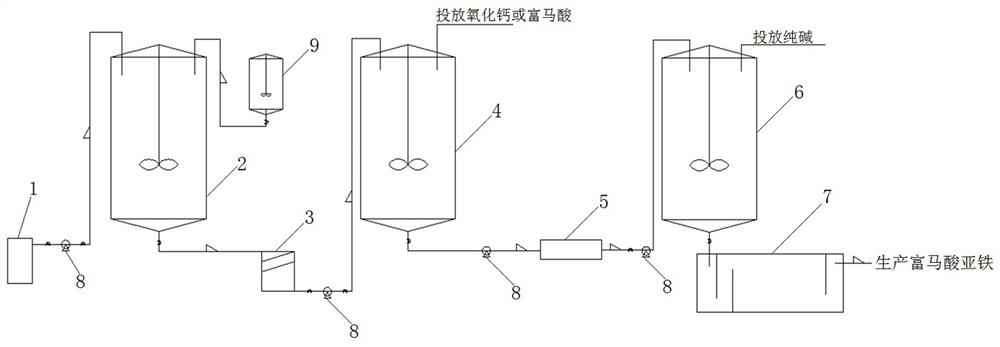
Method and device for treating ferrous fumarate wastewater
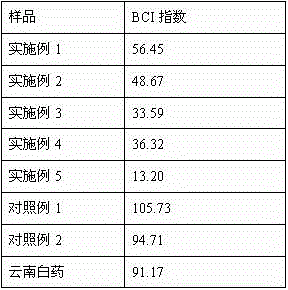
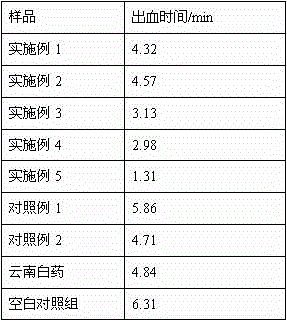
ActiveCN112429882ASolve pollutionShort and efficient processingOrganic compound preparationTreatment involving filtrationWastewaterFERROUS FUMARATE/IRON
Owner:南宁市泽威尔饲料有限责任公司
Pharmaceutical formulation based on ibuprofen and codeine having improved stability
InactiveUS20130064890A1Easy to compressAvoids effect of stickingBiocideNervous disorderSodium fumarateBULK ACTIVE INGREDIENT
Pharmaceutical formulation based on ibuprofen and codeine having improved stability. The invention consists of a novel pharmaceutical formulation having the form of tablets or similar comprising a core composed of an association of ibuprofen and codeine as active ingredients, together with an excipient including at least a diluent, a disintegrating agent, a fluidizing agent and a lubricant which is sodium stearyl fumarate. Said core is coated with a composition based on one or several polymers of diverse modified cellulose ethers and polymers derived from acrylic and methacrylic acids, a plasticiser and, an opacifier or colouring agent and any of the mixtures thereof. These characteristics render the tablets of the invention more efficacious and safe having the form of more stable preparations, without this fact implying greater technological complexity.
Owner:FARMASIERRA MFG
Frozen skipjack block quality improver and preparation method and application thereof
ActiveCN105875796APrevent browningAvoid discoloration and other phenomenaFood ingredient as antioxidantSugar food ingredientsCarrageenanGlycerol
The present invention discloses a frozen skipjack block quality improver. The frozen skipjack block quality improver is prepared from the following components in percentages by mass: 0.01-0.03% of rosemary water extract, 3-5% of white sugar, 0.1-0.3% of sodium fumarate, 3-5% of trehalose, 0.03-0.05% of erythritol, 0.5-1% of carrageenan, 0.03-0.05% of metallothionein, 1-3% of sodium citrate, 3-5% of glycerol, and the balanced being deionized water. The frozen skipjack block quality improver is reasonable and scientific in recipe and good in use safety. Each component is in a synergic function, and the quality improver can effectively avoid browning, color losing and other phenomena of the skipjack blocks, and can greatly improve the freezing quality of the skipjack blocks. The present invention also provides a preparation method of the frozen skipjack block quality improver. The preparation method is simple in steps, has no special requirement for equipment and technology, and is strong in operation, low in preparation costs, and suitable for industrialized large-scale productions.
Owner:ZHEJIANG OCEAN UNIV
Stable Quick Dissolving Dosage Form Comprising Amoxicillin and Clavulanic Acid
ActiveUS20150245995A1Prolong lifeImprove stabilityDispersion deliveryInorganic non-active ingredientsMANNITOL/SORBITOLSodium fumarate
A quick dissolving pharmaceutical formulation is disclosed. In one embodiment, the formulation includes at least the following components: (1) from about 35 to about 50 weight percent amoxicillin or a pharmaceutically acceptable salt thereof; (2) from about 2.0 to about 12 weight percent clavulanic acid or a pharmaceutically acceptable salt thereof; (3) from about 30 to about 40 weight percent mannitol; (4) from about 2 to about 7 weight percent crospovidone; (5) from about 0.5 to about 2.0 weight percent colloidal silicon dioxide; and (6) from about 2.0 to about 5.0 weight percent sodium stearyl fumarate. A method for making a quick dissolving pharmaceutical tablet is also disclosed.
Owner:SANDOZ AG
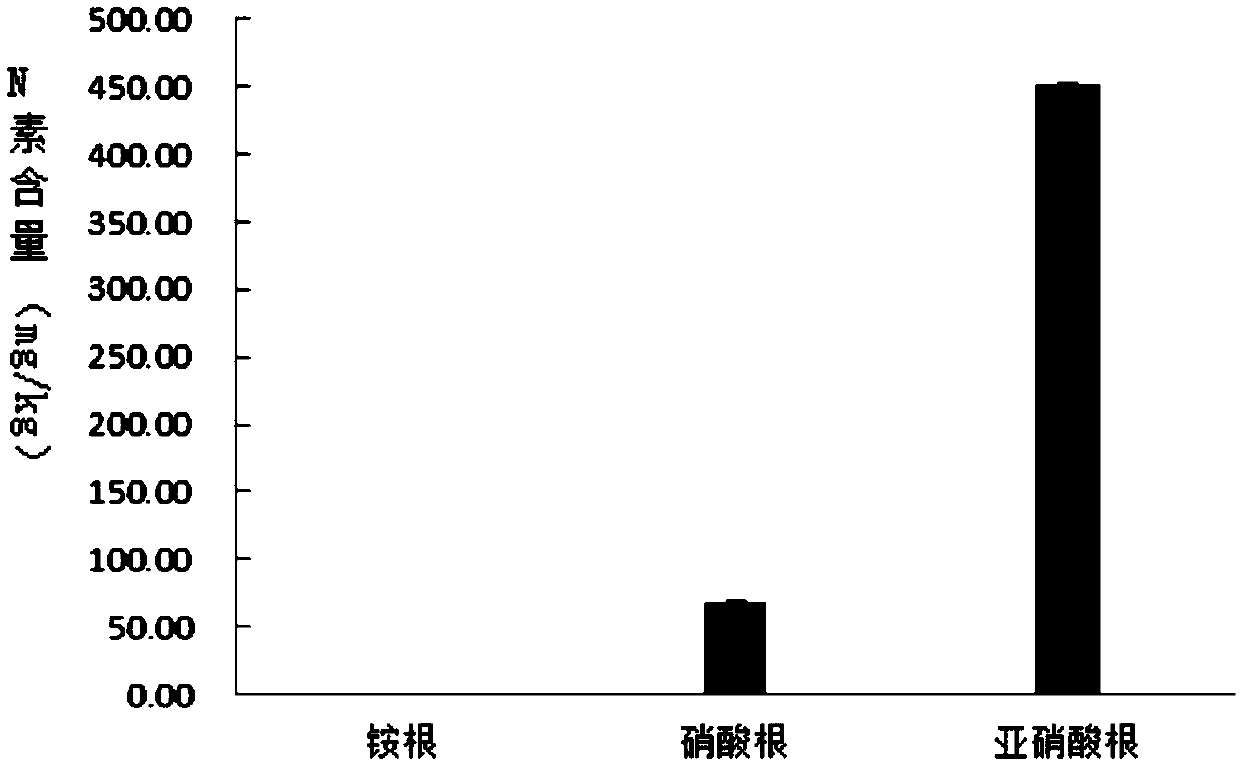
Medium for separating microorganisms in sauerkraut fermentation liquid and preparation method thereof
ActiveCN109593676AThe configuration method is simple and fastBacteriaBiotechnologyIon chromatography
The invention relates to a medium, in particular to a medium for separating microorganisms in sauerkraut fermentation liquid and a preparation method thereof. The preparation method comprises the following steps: selecting sauerkraut fermentation liquid, determining organic acid species of the sample by adoption of high performance liquid chromatography, and then selecting sodium salts corresponding to several organic acids as sources of a medium C; detecting the types of N sources in the sample by adoption of an ion chromatography method and an indophenol blue colorimetric method; and finallydetermining the components of the medium: every 1L of the medium contains 0.1-0.5 g of magnesium sulfate, 0.5-1.0 g of dipotassium phosphate, 0.3-0.9 g of sodium malate, 0.1-0.9 g of sodium oxalate,0.1-0.7 g of sodium fumarate, 0.1-0.5 g of sodium nitrate, 0.1-0.5 g of sodium nitrite and 15-20 g of agar, wherein the pH is 6.8-7. The medium is simple and convenient in preparation method and provides a new medium for screening functional microorganisms.
Owner:JIANGSU UNIV
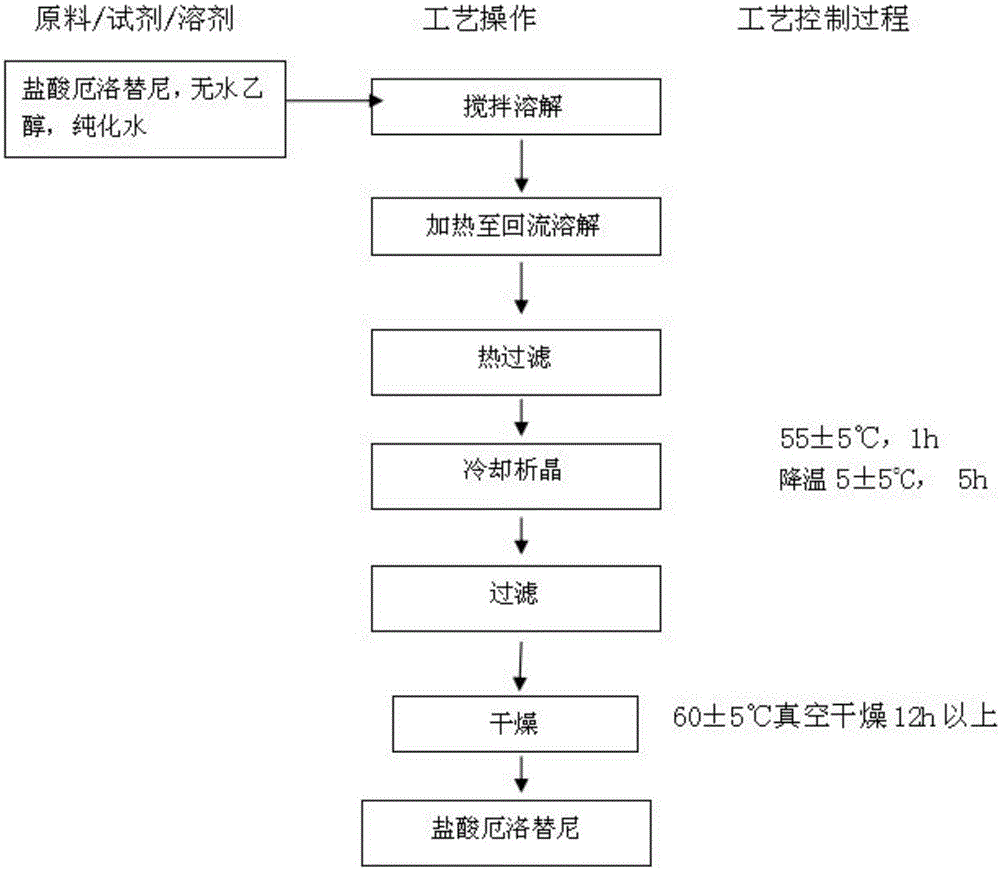
Erlotinib hydrochloride composition tablet and preparation method thereof
InactiveCN105748429AThe preparation process route is stableEasy to operateOrganic active ingredientsOrganic chemistry methodsCelluloseCyclodextrin
The invention discloses an erlotinib hydrochloride composition tablet and a preparation method thereof. The erlotinib hydrochloride composition tablet comprises the following ingredients in percentage by weight: 20-38% of erlotinib hydrochloride crystals, 30-50% of pregelatinized starch, 25-45% of calcium sulfate, 1-5% of ethyl cellulose, 6-12% of beta-cyclodextrin, 0.2-3% of Tween, 5-10% of crosslinked sodium carboxymethyl starch, 0.5-1.5% of sodium stearyl fumarate and 0.2-2% of micropowder silica gel. A preparation process route of the erlotinib hydrochloride composition tablet is stable, simple and easy to operate, set technological parameters can effectively control each reaction, and sample detection results show that an erlotinib hydrochloride crude drug is stable in crystal form and relatively good in purity and quality requirements of a final product can be met. A synthesis and production technology of the erlotinib hydrochloride composition tablet is simple, stable and feasible and is applicable to mass production; and quality is controllable, and stability is relatively good.
Owner:DEYANG HUAKANG PHARMA
Composition containing collagen and preparation method of composition containing collagen
The invention relates to a composition containing collagen and a preparation method of the composition containing collagen, and belongs to the field of drugs and health-care products. The compositioncontaining collagen basically consists of 15-1200 parts by weight of collagen, 50-1000 parts by weight of ferment, 200-900 parts by weight of an excipient and 5-30 parts by weight of a lubricant, wherein the excipient is selected from one or more of sorbitol, mannitol, lactose and soluble starch; and the lubricant is selected from one or more of stearic acid, micronization poloxamer, sodium stearyl fumarate, magnesium stearate, talcum powder, micro powder silica gel, silicon dioxide, PEG6000, sodium fumarate, magnesium laurylsulfate and sldium lauryl sulfate. The composition disclosed by the invention is prepared into a solid preparation rather than a liquid preparation, so that the situation that the collagen existing in a liquid preparation formulation is invalid and degraded during long-term storage is avoided, and collagen products in a solid preparation formulation have the advantage of being convenient to store, transport, carry and take.
Owner:BEIJING KANGLIJI BIOLOGICAL TECH CO LTD
Rivaroxaban pharmaceutical composition
ActiveCN112656772AImprove stabilityHigh dissolution rateOrganic active ingredientsPharmaceutical delivery mechanismCoated tabletsActive agent
The invention relates to a pharmaceutical composition taking rivaroxaban as a main drug component and a preparation method of the pharmaceutical composition. Specifically, the invention relates to a pharmaceutical composition taking rivaroxaban as the main drug component, and the composition can be further prepared into a film-coated tablet as a specific administration form through a certain preparation process. By applying a method of a specific preparation process, the use of a surfactant in the preparation is avoided, and meanwhile, the dissolution rate of the tablet is effectively improved. And the sodium stearoyl fumarate is used in the composition, so that the significant increase of the degradation impurity I in the long-term storage process of the tablet is effectively avoided. Accelerated stability research finds that the rivaroxaban-containing film-coated tablet prepared by the prescription and the process steps disclosed by the invention is stable and controllable in quality.
Owner:浙江东日药业有限公司
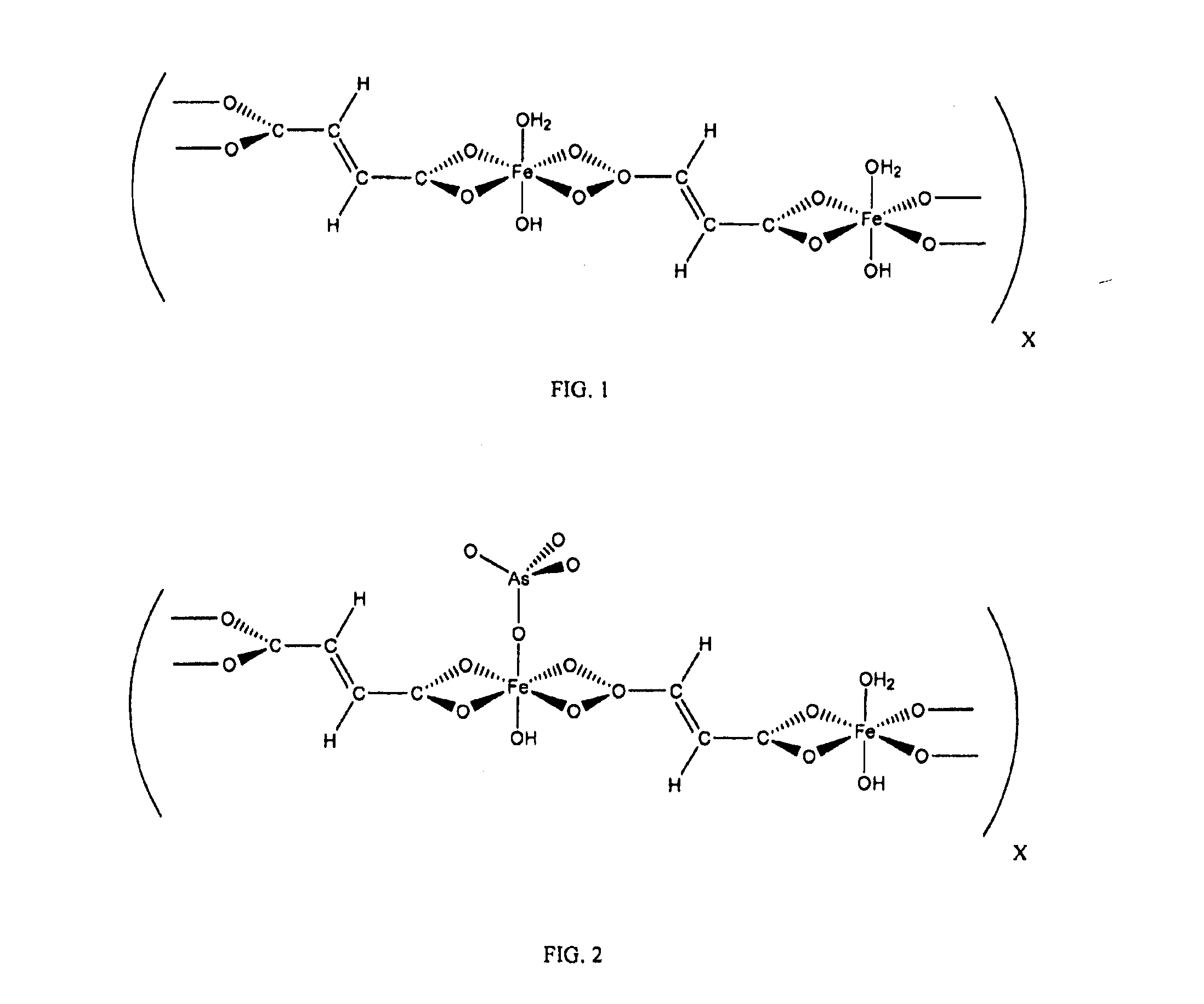
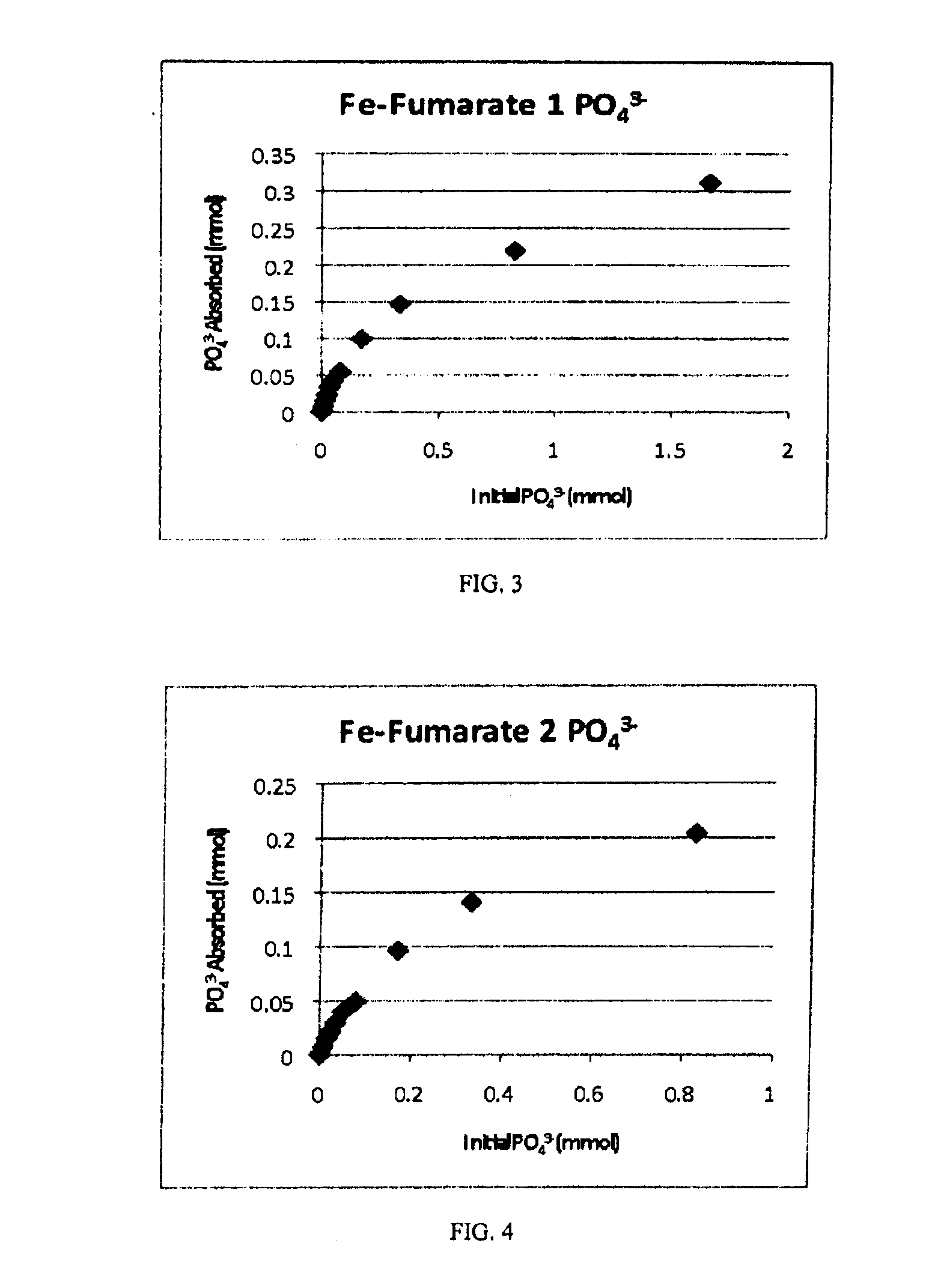
Iron coordination polymers for adsorption of arsenate and phosphate
A method includes combining an aqueous solution of sodium fumarate with an aqueous solution of iron chloride to form a mixture, and obtaining an iron coordination polymer as an amorphous compound formed as a precipitate from the mixture. The iron coordination polymer may be used to bind contaminants, such as arsenate and phosphate from water.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Penetrating agent capable of purifying environment for leather and preparation method of penetrating agent
The invention discloses a penetrating agent capable of purifying environment for leather and a preparation method of the penetrating agent. The penetrating agent capable of purifying the environment for leather is prepared from the following raw materials in parts by weight: 50-60 parts of sodium lauryl polyoxyethylene ether sulphate, 15-20 parts of citric acid, 4-6 parts of dimethyltin bis(2-ethylhexylmercaptoacetate), 6-8 parts of sodium fumarate, 3-4 parts of benzoyl chloride, 6-8 parts of lecithin, 2-4 parts of glyceryl triacetate, 9-10 parts of microcrystalline cellulose, 2-4 parts of lignosulfonate, 1-3 parts of amino-2-methyl-1-propyl alcohol, 8-10 parts of modified moss and 35-40 parts of water. The penetrating agent capable of purifying the environment for leather has the advantages that raw materials are safe and reliable and pollution-free, durability and broad applicability are good, the sterilizing, dedusting and odour removing effects are obvious, the environment can be effectively purified, the overall performance of the penetrating agent can be effectively improved by virtue of modified moss, the levelling dyeing property is excellent, and the penetration performance of leather dye can be obviously improved.
Owner:HEFEI ANSHAN COATING FABRICS
Acarbose capsule and preparation method thereof
ActiveCN104546795AStrong hydrolase inhibitory abilityOrganic active ingredientsMetabolism disorderPolyethylene glycolMagnesium stearate
The invention relates to an acarbose capsule and a preparation method thereof. Specifically, the capsule comprises a capsule shell and filler which is coated in the capsule shell and is in the form of powder or granule, wherein the filler consists of an active substance acarbose, a thinner, a lubricant and a flow aid; the thinner is selected from starch, dextrin, carboxymethyl starch, hydroxypropyl starch, modified starch, modified pregelatinized starch, microcrystalline cellulose, calcium phosphate, calcium hydrogen phosphate, calcium carbonate and the like and the combinations thereof; the lubricant is selected from magnesium stearate, stearic acid, polyethylene glycol with molecular weight of 4000-8000, calcium stearate, zinc stearate, octadecyl sodium fumarate and the combinations thereof. The capsule disclosed by the invention is excellent in pharmaceutical properties such as excellent stability.
Owner:HANGZHOU ZHUYANGXIN PHARMA
Method for fermenting and producing L-malic acid with raw material of citric acid broth
ActiveCN102242160AIncrease productionImprove conversion rateMicroorganism based processesOxidoreductasesFumaraseSodium fumarate
The invention discloses a method for fermenting and producing L-malic acid with a raw material of citric acid broth. According to the method, citric acid broth is carbon source, through fermentation fumarase is generated, and by utilizing broth of fumarase and an enzyme conversion method, sodium fumarate generates the L-malic acid. After conversion of 20 to 26 hours, malic acid content reaches 78-85g / L, and conversion rate reaches 81.2%-85.5%. The method of the invention has the advantages of simple technology, low energy consumption, cheap raw material and substantial saving of production cost.
Owner:ANHUI BBCA FERMENTATION TECH ENG RES
Preservative for maritime iced preservation of shrimps and preparation method and application thereof
ActiveCN104872270AThe formula is scientific and reasonableLow costMeat/fish preservation using chemicalsAdditive ingredientSodium Caseinate
The invention discloses a preservative for maritime iced preservation of shrimps. The preservative is composed of the following ingredients, by weight, 0.5-1% of glucose oxidase, 1-3% of glucose, 10-15% of dithiothreitol, 0.75-1% of nisin, 20-30% of sodium caseinate, 3-5% of metallothionein, 20-30% of sodium fumarate and the rest being rosmarinic acid. The formula of the preservative is scientific, reasonable and low-cost. Through synergism of the ingredients, the preservative has excellent antibacterial, fresh-keeping and anti-blackening effects. By the use of the preservative, freshness lifetime of shrimps can be prolonged, quality of shrimps is good, and the problem that freshness lifetime of shrimps is short, shrimp bodies are easy to dehydrate and quality is easy to reduce during the iced preservation process of shrimps is effectively solved. The preservative provided by the invention has a wide application prospect. The invention also discloses a preparation method of the preservative for maritime iced preservation of shrimps. The preservative can be obtained by uniformly mixing the ingredients after weighing in proportion. The preparation steps are simple; investment in equipment is less; and cost is low. The preservative is suitable for large-scale industrial production.
Owner:MARINE FISHERIES RES INST OF ZHEJIANG
Solid pharmaceutical composition for treating mental diseases
PendingCN113855640AImprove performanceNervous disorderPharmaceutical non-active ingredientsCompulsive disordersStarch corn
The invention relates to a solid pharmaceutical composition for treating mental diseases, in particular to a method for preparing a fluvoxamine maleate tablet. The fluvoxamine maleate tablet is prepared from fluvoxamine maleate, mannitol, microcrystalline cellulose, pregelatinized starch, corn starch, silicon dioxide and sodium stearyl fumarate. The method comprises the following steps that the materials are mixed and granulated, and then pressed into tablets on a tablet press. The invention further relates to the tablets prepared by the method, the coated tablets prepared by taking the tablets as tablet cores, and application of the tablets and the coated tablets in preparation of medicines for treating mental diseases, such as depression and related symptoms, obsessive-compulsive disorder. The tablets have excellent performance.
Owner:HUNAN DONGTING PHARMA
Functional auxiliary material as well as preparation method and application thereof
InactiveCN102961752AGood hygroscopicityExcellent moisture absorptionPharmaceutical non-active ingredientsFood preparationSodium fumarateSpray drying
Owner:苏州艾费堂医药科技有限公司
Domperidone tablet and preparation method thereof
ActiveCN112168795AReduce lossGood content uniformityOrganic active ingredientsDigestive systemSodium fumarateStearic acid
The invention relates to the technical field of pharmaceutical preparations, and particularly relates to a domperidone tablet and a preparation method thereof. The domperidone tablet is prepared fromthe following components in parts by weight: 1 part of domperidone, 3 to 10 parts of diluent A, 0.5 to 3.5 parts of diluent B, 0.1 to 0.9 part of disintegrating agent, 0.3 to 1.0 part of adhesive, 0.004 to 0.02 part of solubilizer, 0.05 to 0.15 part of lubricant A and 0.03 to 0.13 part of lubricant B, wherein the lubricant A is at least one of silicon dioxide, sodium stearyl fumarate and talcum powder; the lubricant B is magnesium stearate. The domperidone tablet has the advantages of low main drug loss, high content uniformity, excellent dissolution performance and excellent appearance.
Owner:四川维奥制药有限公司
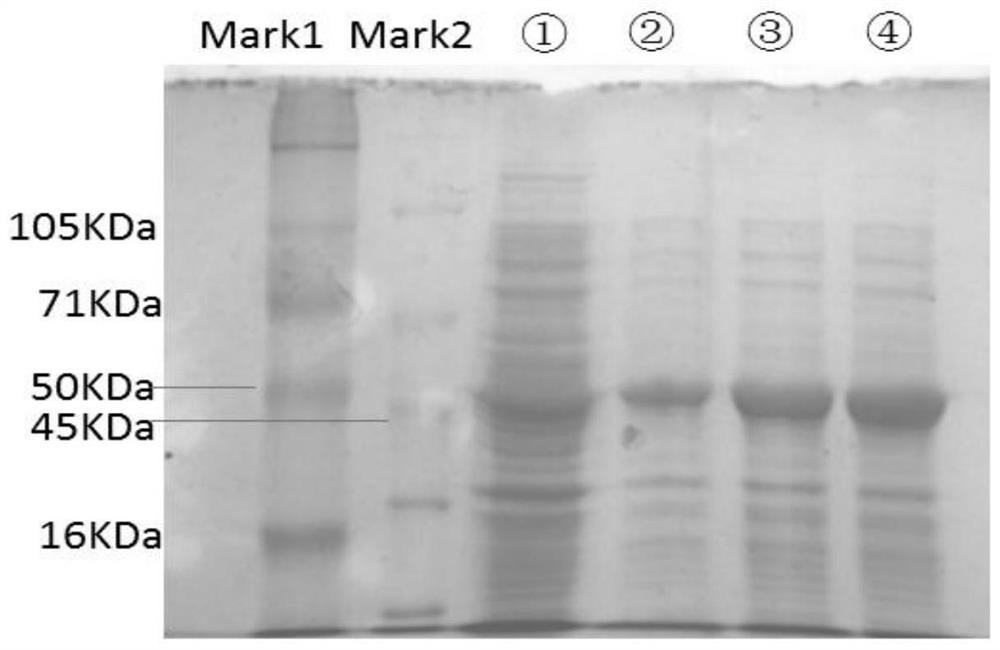
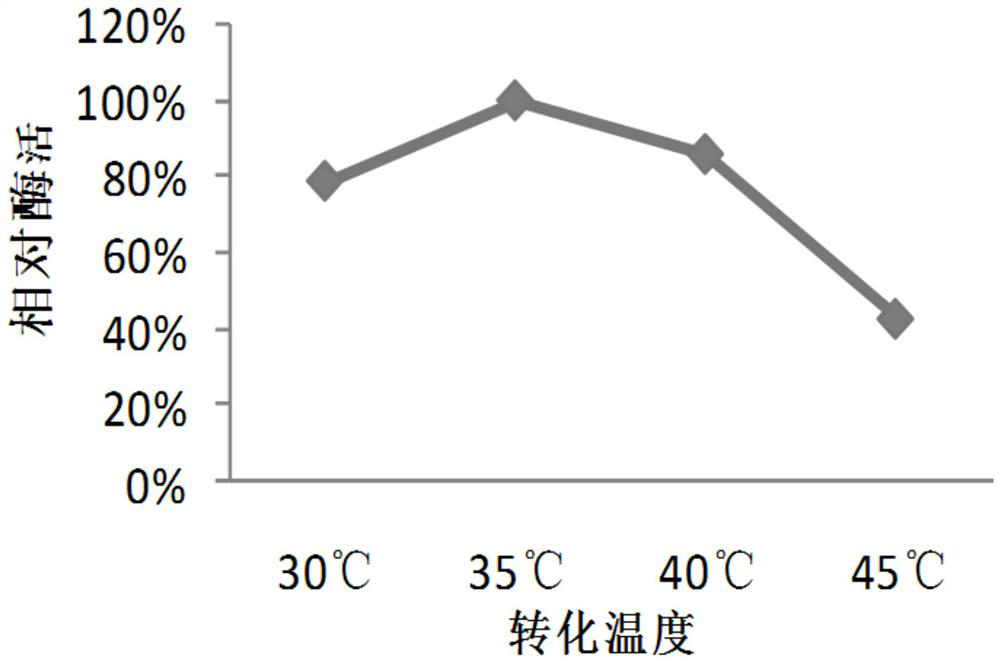
Method for synthesizing L-malic acid through whole-cell catalysis of recombinant escherichia coli
The invention belongs to the technical field of biological enzyme synthesis, and particularly relates to a method for synthesizing L-malic acid through whole-cell catalysis of recombinant escherichia coli. The invention aims to solve the technical problems of low yield and high cost of fumarase extraction. According to the technical scheme, the method for synthesizing the L-malic acid through the recombinant escherichia coli specifically comprises the following steps: constructing escherichia coli engineering bacteria for expressing a fumarase gene, performing liquid culture on the engineering bacteria, adding a reaction substrate sodium fumarate, performing whole-cell catalysis until the reaction is complete, and collecting reaction liquid. According to the application, L-malic acid is produced by using sodium fumarate as a conversion substrate through recombinant escherichia coli whole cells by utilizing a molecular biological technology. The yield of the L-malic acid in the method can reach 95%. The invention provides a direction for the research of increasing the yield of malic acid and lays a foundation for the research of other organic acids.
Owner:TAIZHOU UNIV
Compound chloramphenicol vaginal swelling suppository, as well as preparation method and detection method thereof
ActiveCN103768095AGuaranteed effective concentrationPrevent outflowWeighing by removing componentInorganic boron active ingredientsSecondary InfectionsVaginal swelling
The invention provides a compound chloramphenicol vaginal swelling suppository, as well as a preparation method and a detection method thereof. The swelling suppository comprises a drug-containing matrix formed by an active component and a matrix, and a swelling vector, wherein the active component comprises chloramphenicol, boric acid, vitamin A and vitamin D2; the drug-containing matrix also comprises octadecyl sodium fumarate, sucrose monopalmitate and polyoxyethylenealiphatic alcohol ether; the layer out of the core of the swelling suppository is coated with an ointment layer. The compound chloramphenicol vaginal swelling suppository has the beneficial effects of high stability and small vaginal irritation, adopts seven original advanced technologies, has the beneficial effects of preventing a liquid medicine from overflowing, preventing secondary infection and the like, and is quick in response and long in curative effect.
Owner:哈尔滨田美药业股份有限公司
Low-cost preparation method of pharmaceutical adjuvant sodium stearyl fumarate
ActiveCN112624919AReduce consumptionReduce manufacturing costOrganic compound preparationOrganic chemistry methodsPtru catalystOrganic solvent
The invention belongs to the technical field of preparation of pharmaceutic adjuvants, and discloses a low-cost preparation method of a pharmaceutical adjuvant sodium stearyl fumarate. The method comprises the following steps: adding octadecanol and maleic anhydride into an organic solvent, performing heating to 90-130DEG C, carrying out a stirring reaction to obtain monooctadecanol maleate, adding a solid isomerization catalyst, continuously carrying out a heat preservation stirring reaction at 90-130DEG C to obtain monooctadecanol fumarate, performing filtering to separate a solid isomerization catalyst in the system, adding an alkali solution into the reaction system, carrying out stirring reaction at the temperature of 35-55DEG C, and separating and purifying a product to obtain the sodium stearyl fumarate. The solid isomerization catalyst and a one-pot method are adopted for synthesis, after catalysis is completed, separation can be conducted from a reaction system in a simple filtering mode, the production process is simplified, the production cost is reduced, and the product quality is high.
Owner:南京紫鸿生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com