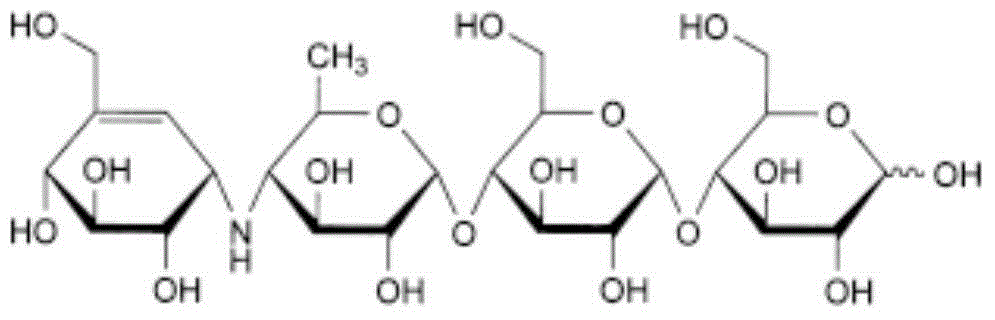
Acarbose capsule and preparation method thereof
A technology of acarbose and capsules, applied in the field of medicine, can solve the problems such as the inability to improve the fluidity, the easy moisture absorption of acarbose, and the powder spraying.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: Preparation of acarbose capsules
[0095] formula:
[0096] Acarbose
50mg
20mg
90mg
Modified pregelatinized starch
40mg
Sodium octadecyl fumarate
1.5mg
silica
1mg
[0097] Preparation method:
[0098] (1) Make the acarbose that crosses 80 mesh sieves through being pulverized and mix evenly with the lubricant of prescription quantity;
[0099] (2) mix the diluent and the mixed powder obtained in step (1) through pulverizing in advance and passing through an 80-mesh sieve;
[0100] (3) mixing the glidant and the mixed powder obtained in step (2) in a three-dimensional mixer for 5-10 minutes to obtain a uniform total mixture, which is used as a filler for capsules;
[0101] (4) Detect the content of the active substance in the filler, and calculate the loading amount of the filler in each capsule;
[0102] (5) Filling the obtained filler into...
Embodiment 2
[0103] Embodiment 2: preparation of acarbose capsules
[0104] formula:
[0105] Acarbose
50mg
20mg
Cassava starch
87.5mg
Modified pregelatinized starch
40mg
Sodium octadecyl fumarate
1.5mg
silica
1mg
[0106] Preparation method:
[0107] (1) Make the acarbose that crosses 80 mesh sieves through being pulverized and mix evenly with the lubricant of prescription quantity;
[0108] (2) mix the diluent and the mixed powder obtained in step (1) through pulverizing in advance and passing through an 80-mesh sieve;
[0109] (3) mixing the glidant and the mixed powder obtained in step (2) in a three-dimensional mixer for 5-10 minutes to obtain a uniform total mixture, which is used as a filler for capsules;
[0110] (4) Detect the content of the active substance in the filler, and calculate the loading amount of the filler in each capsule;
[0111] (5) Filling the obtained fille...
Embodiment 3
[0112] Embodiment 3: Preparation of acarbose capsules
[0113] formula:
[0114] Acarbose
50mg
15mg
95mg
Modified pregelatinized starch
45mg
Sodium octadecyl fumarate
1.2mg
silica
0.5mg
[0115] Preparation method:
[0116] (1) Make the acarbose that crosses 80 mesh sieves through being pulverized and mix evenly with the lubricant of prescription quantity;
[0117] (2) mix the diluent and the mixed powder obtained in step (1) through pulverizing in advance and passing through an 80-mesh sieve;
[0118] (3) mixing the glidant and the mixed powder obtained in step (2) in a three-dimensional mixer for 5-10 minutes to obtain a uniform total mixture, which is used as a filler for capsules;
[0119] (4) Detect the content of the active substance in the filler, and calculate the loading amount of the filler in each capsule;
[0120] (5) Filling the obtained filler...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com