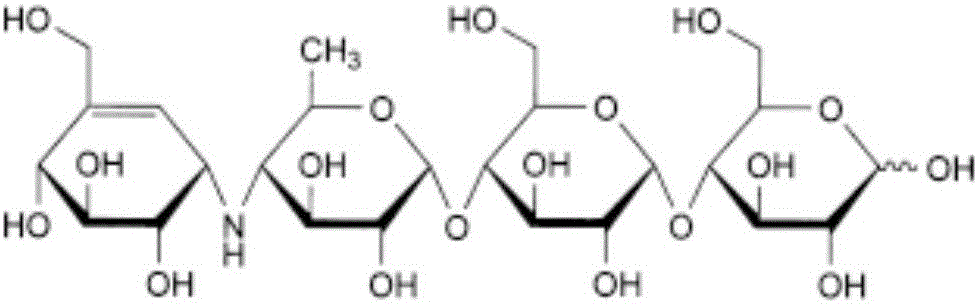
Acarbose capsule pharmaceutical composition
A technology of acarbose and capsules, applied in the field of medicine, can solve the problems of inconvenience in taking, large differences, poor fluidity of acarbose and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: Preparation of acarbose capsules
[0100] formula:
[0101] Acarbose 50mg microcrystalline cellulose 20mg corn starch 90mg Modified pregelatinized starch 40mg silica 1mg Magnesium stearate 1.5mg
[0102] Preparation method:
[0103] (1) Mix acarbose and the microcrystalline cellulose of the prescribed amount evenly, and spray concentration>98% ethanol (the weight ratio of acarbose to the ethanol is 50:4) into the mixed powder under high-speed mixing state. ), the resulting mixture is pre-compressed into tablets with an extruder, and then the tablets are broken into granules, dried to remove ethanol, and then the granules are pulverized into fine powders crossing 80 mesh sieves;
[0104] (2) Mix other diluents that have been pulverized and crossed 80 mesh sieves with the mixed powder obtained in step (1) in advance;
[0105] (3) mixing the glidant and lubricant with the mixed powder obtained in step (2)...
Embodiment 2
[0108] Embodiment 2: preparation of acarbose capsules
[0109] formula:
[0110] Acarbose 50mg microcrystalline cellulose 20mg Cassava starch 87.5mg Modified pregelatinized starch 40mg silica 1mg Magnesium stearate 1.5mg
[0111] Preparation method:
[0112] (1) Mix acarbose and the microcrystalline cellulose of the prescribed amount evenly, and spray concentration>98% ethanol (the weight ratio of acarbose to the ethanol is 50:3) into the mixed powder under high-speed mixing state ), the resulting mixture is pre-compressed into tablets with an extruder, and then the tablets are broken into granules, dried to remove ethanol, and then the granules are pulverized into fine powders crossing 80 mesh sieves;
[0113] (2) Mix other diluents that have been pulverized and crossed 80 mesh sieves with the mixed powder obtained in step (1) in advance;
[0114] (3) mixing the glidant and lubricant with the mixed powder obtained in ste...
Embodiment 3
[0117] Embodiment 3: Preparation of acarbose capsules
[0118] formula:
[0119] Acarbose 50mg microcrystalline cellulose 15mg Potato starch 95mg Modified pregelatinized starch 45mg silica 0.5mg Magnesium stearate 1.2mg
[0120] Preparation method:
[0121] (1) Mix acarbose and the microcrystalline cellulose of the prescribed amount evenly, and spray ethanol with a concentration>98% into the mixed powder under high-speed mixing state (the weight ratio of acarbose and the ethanol is 50:2 ), the resulting mixture is pre-compressed into tablets with an extruder, and then the tablets are broken into granules, dried to remove ethanol, and then the granules are pulverized into fine powders crossing 80 mesh sieves;
[0122] (2) Mix other diluents that have been pulverized and crossed 80 mesh sieves with the mixed powder obtained in step (1) in advance;
[0123] (3) mixing the glidant and lubricant with the mixed powder obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com