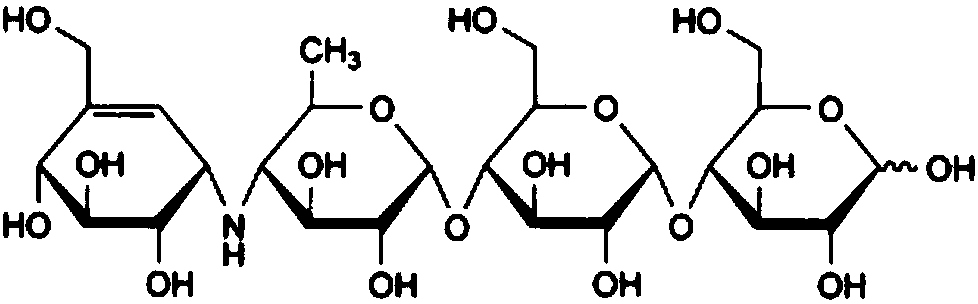
Acarbose Capsules and Preparation Method
A technology of acarbose and capsules, which is applied in the field of medicine and can solve the problems of acarbose, such as easy moisture absorption, poor fluidity, and unqualified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: Preparation of acarbose capsules
[0095] formula:
[0096] Acarbose
50mg
20mg
90mg
Modified pregelatinized starch
40mg
Sodium Octadecyl Fumarate
1.5mg
silica
1mg
[0097] Preparation method:
[0098] (1) Make the acarbose that crosses 80 mesh sieves through being pulverized and mix evenly with the lubricant of prescription quantity;
[0099] (2) mix the diluent and the mixed powder obtained in step (1) through pulverizing in advance and passing through an 80-mesh sieve;
[0100] (3) mixing the glidant and the mixed powder obtained in step (2) in a three-dimensional mixer for 5-10 minutes to obtain a uniform total mixture, which is used as a filler for capsules;
[0101] (4) Detect the content of the active substance in the filler, and calculate the loading amount of the filler in each capsule;
[0102] (5) Filling the obtained filler into t...
Embodiment 2
[0103] Embodiment 2: preparation of acarbose capsules
[0104] formula:
[0105] Acarbose
50mg
20mg
Cassava starch
87.5mg
Modified pregelatinized starch
40mg
Sodium Octadecyl Fumarate
1.5mg
silica
1mg
[0106] Preparation method:
[0107] (1) Make the acarbose that crosses 80 mesh sieves through being pulverized and mix evenly with the lubricant of prescription quantity;
[0108] (2) mix the diluent and the mixed powder obtained in step (1) through pulverizing in advance and passing through an 80-mesh sieve;
[0109] (3) mixing the glidant and the mixed powder obtained in step (2) in a three-dimensional mixer for 5-10 minutes to obtain a uniform total mixture, which is used as a filler for capsules;
[0110] (4) Detect the content of the active substance in the filler, and calculate the loading amount of the filler in each capsule;
[0111] (5) Filling the obtained filler ...
Embodiment 3
[0112] Embodiment 3: Preparation of acarbose capsules
[0113] formula:
[0114] Acarbose
50mg
15mg
95mg
Modified pregelatinized starch
45mg
Sodium Octadecyl Fumarate
1.2mg
silica
0.5mg
[0115] Preparation method:
[0116] (1) Make the acarbose that crosses 80 mesh sieves through being pulverized and mix evenly with the lubricant of prescription quantity;
[0117] (2) mix the diluent and the mixed powder obtained in step (1) through pulverizing in advance and passing through an 80-mesh sieve;
[0118] (3) mixing the glidant and the mixed powder obtained in step (2) in a three-dimensional mixer for 5-10 minutes to obtain a uniform total mixture, which is used as a filler for capsules;
[0119] (4) Detect the content of the active substance in the filler, and calculate the loading amount of the filler in each capsule;
[0120] (5) Filling the obtained filler i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com