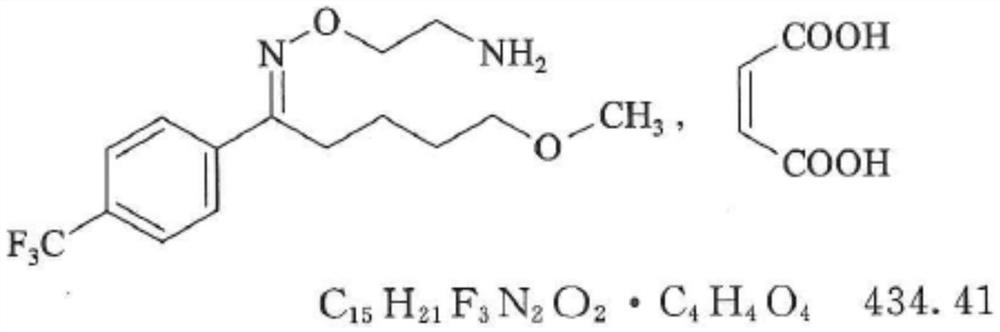
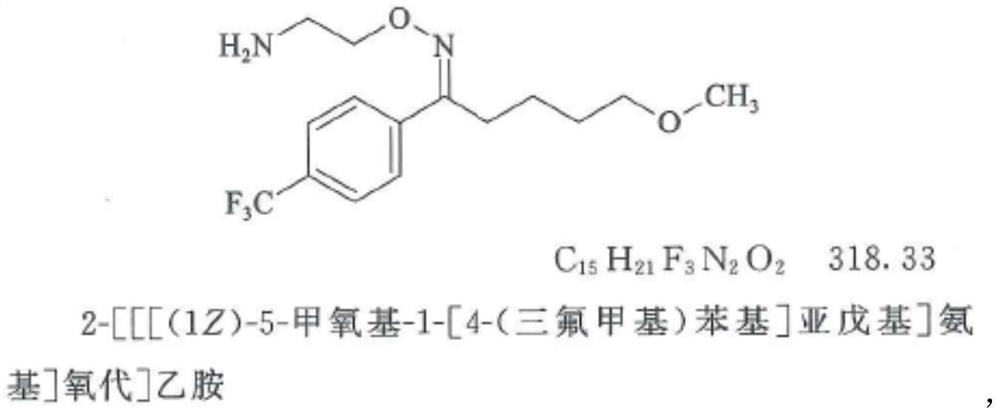
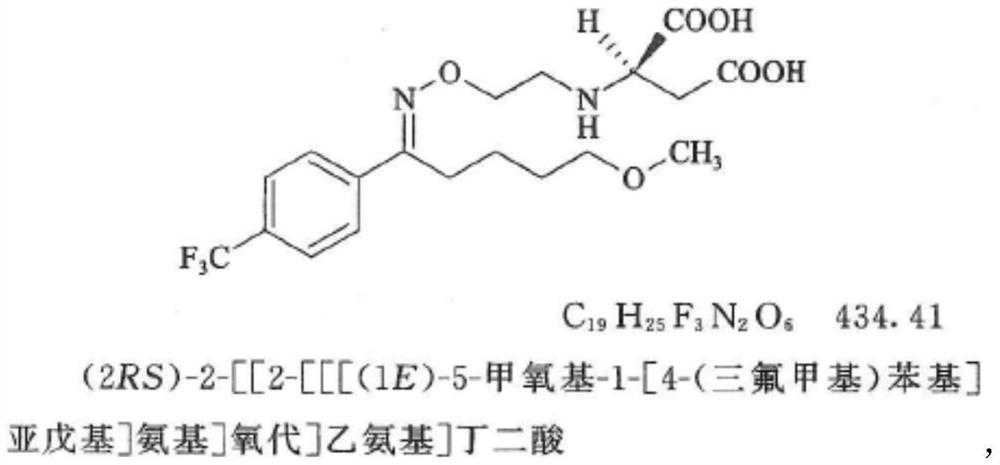
Solid pharmaceutical composition for treating mental diseases
A material and high-speed mixing technology, applied in the field of medicine, can solve the problems of small interfering impurities, easy interference of impurity III, lack of accuracy of impurity IV, etc., and achieve the effect of excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: prepare fluvoxamine maleate sheet (plain sheet)
[0096] prescription:
[0097] Fluvoxamine maleate (Lot01): 50 parts by weight,
[0098] Mannitol: 55 parts by weight,
[0099] Microcrystalline cellulose: 35 parts by weight,
[0100] Pregelatinized starch: 5 parts by weight,
[0101] Corn starch: 20 parts by weight,
[0102] Silica: 2 parts by weight,
[0103] Sodium stearyl fumarate: 5 parts by weight.
[0104] Preparation method:
[0105] (1) Each material is crushed in advance to pass through a 120-mesh sieve;
[0106] (2) Fluvoxamine maleate, mannitol, and microcrystalline cellulose are put in the pot of the high-speed mixing granulator, and mixed uniformly;
[0107] (3) Mix the pregelatinized starch with purified water (the amount of water is 15 times that of the pregelatinized starch) evenly, heat to 75-85°C under stirring and keep it warm for more than 30min to obtain a binder solution;
[0108] (4) Slowly add the binder solution to a hig...
Embodiment 2
[0112] Embodiment 2: preparation fluvoxamine maleate sheet (plain sheet)
[0113] prescription:
[0114] Fluvoxamine maleate (Lot01): 50 parts by weight,
[0115] Mannitol: 60 parts by weight
[0116] Microcrystalline cellulose: 30 parts by weight
[0117] Pregelatinized starch: 5.5 parts by weight,
[0118] Corn starch: 15 parts by weight,
[0119] Silica: 2.5 parts by weight,
[0120] Sodium stearyl fumarate: 4 parts by weight.
[0121] Preparation method:
[0122] (1) Each material is crushed in advance to pass through a 120-mesh sieve;
[0123] (2) Fluvoxamine maleate, mannitol, and microcrystalline cellulose are put in the pot of the high-speed mixing granulator, and mixed uniformly;
[0124] (3) Mix pregelatinized starch with purified water (the amount of water is 12 times the amount of pregelatinized starch), mix evenly, heat to 75-85°C under stirring and keep it warm for more than 30min to obtain a binder solution;
[0125](4) Slowly add the binder solution ...
Embodiment 3
[0129] Embodiment 3: preparation fluvoxamine maleate sheet (plain sheet)
[0130] prescription:
[0131] Fluvoxamine maleate (Lot01): 50 parts by weight,
[0132] Mannitol: 50 parts by weight
[0133] Microcrystalline cellulose: 40 parts by weight,
[0134] Pregelatinized starch: 4.5 parts by weight,
[0135] Corn starch: 25 parts by weight,
[0136] Silica: 1.5 parts by weight,
[0137] Sodium stearyl fumarate: 6 parts by weight.
[0138] Preparation method:
[0139] (1) Each material is crushed in advance to pass through a 120-mesh sieve;
[0140] (2) Fluvoxamine maleate, mannitol, and microcrystalline cellulose are put in the pot of the high-speed mixing granulator, and mixed uniformly;
[0141] (3) Mix the pregelatinized starch with purified water (the amount of water is 18 times the amount of pregelatinized starch), mix evenly, heat to 75-85°C under stirring and keep it warm for more than 30min to obtain a binder solution;
[0142] (4) Slowly add the binder sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com