Low-cost preparation method of pharmaceutical adjuvant sodium stearyl fumarate
A technology of sodium stearyl fumarate and pharmaceutical excipients, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, and organic chemical methods, and can solve problems such as unfavorable preparation processes, high preparation costs, and increased consumption of alkali solutions , to achieve high catalytic efficiency, high product quality, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) PS microspheres with an average particle size of 10 μm were added to concentrated sulfuric acid, reacted at a temperature of 60 °C and stirred for 12 h, the reaction product was washed with water and anhydrous ethanol in turn, and dried in vacuum at a temperature of 60 °C to obtain Sulfonated polystyrene microspheres; the obtained sulfonated polystyrene microspheres were added to a 20% mass concentration of aluminum chloride ethanol solution for ultrasonic treatment for 2 h to carry out adsorption and immobilization reaction, washed with ethanol, and vacuum dried at 60°C to obtain sulfonation Aluminum trichloride supported on polystyrene microspheres.
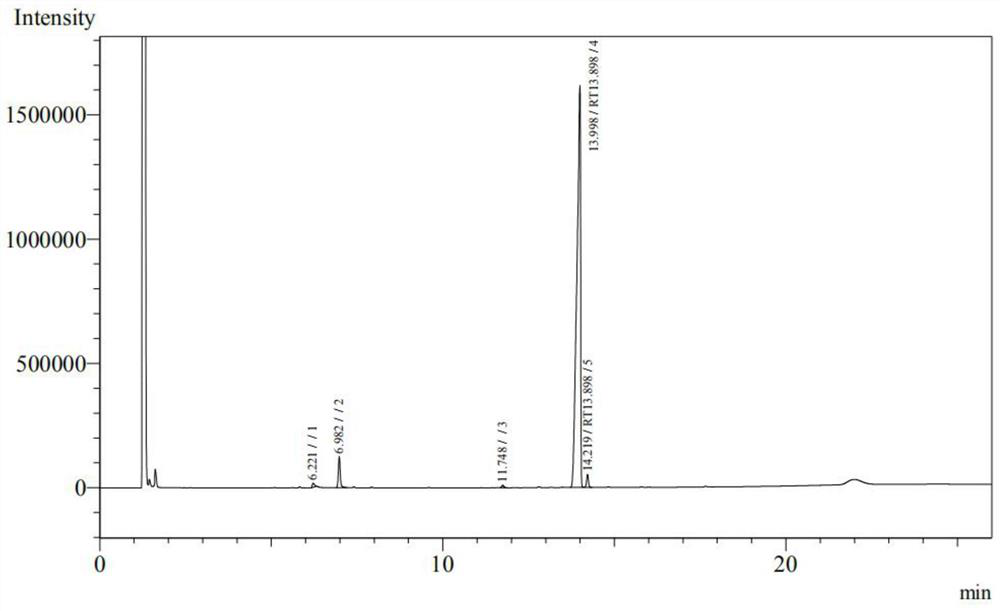
[0047] (2) adding stearyl alcohol to maleic anhydride in a molar ratio of 1:1.02 into a toluene solvent, heating to 120-130° C., stirring and refluxing for 4 hours to obtain monostearyl maleate. The detected yield is 95%, and the product is detected according to the Chinese Pharmacopoeia CP2020 version. The peak time...
Embodiment 2
[0052] (1) PS microspheres with an average particle size of 50 μm were added to concentrated sulfuric acid, reacted at a temperature of 80 °C for 8 h under stirring conditions, the reaction product was washed with water and anhydrous ethanol in turn, and dried in vacuum at a temperature of 80 °C to obtain Sulfonated polystyrene microspheres; the obtained sulfonated polystyrene microspheres were added to a 10% mass concentration of aluminum chloride ethanol solution for ultrasonic treatment for 4 hours to carry out adsorption and immobilization reaction, washed with ethanol, and vacuum dried at 80°C to obtain sulfonation Aluminum trichloride supported on polystyrene microspheres.
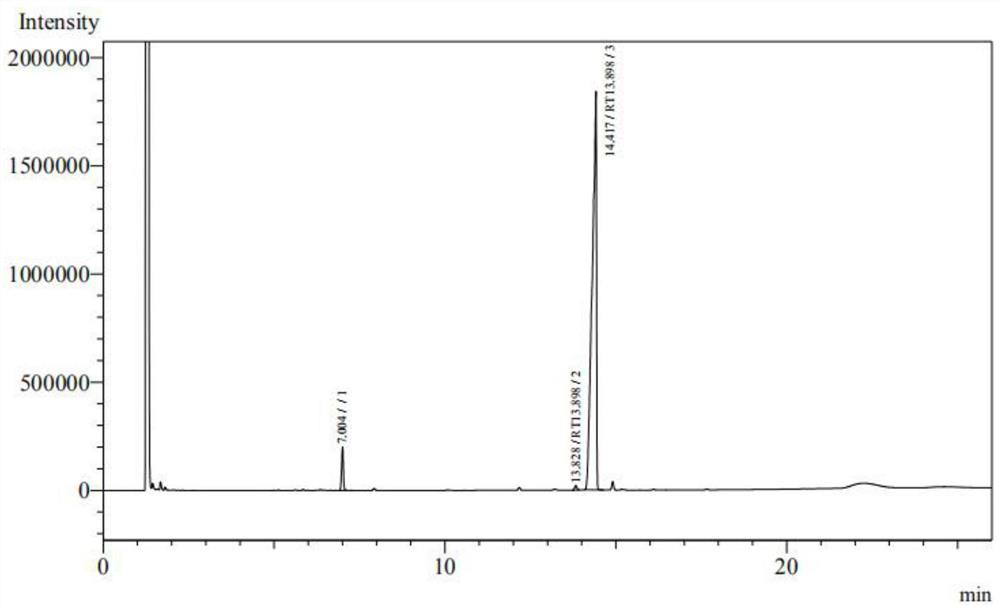
[0053] (2) adding stearyl alcohol and maleic anhydride in a molar ratio of 1:1.05 to a toluene solvent, heating to 120-130° C., stirring and refluxing for 5 hours to obtain monostearyl maleate. The detected yield was 97.5%, and the product was detected according to the Chinese Pharmacopoeia CP2020 ve...
Embodiment 3
[0058] (1) PS microspheres with an average particle size of 80 μm were added to concentrated sulfuric acid, reacted at a temperature of 40 °C and stirring for 24 h, the reaction product was washed with water and anhydrous ethanol in turn, and vacuum-dried at a temperature of 80 °C to obtain Sulfonated polystyrene microspheres; the obtained sulfonated polystyrene microspheres were added to a 20% mass concentration of aluminum chloride ethanol solution for ultrasonic treatment for 4 h to carry out adsorption and immobilization reaction, washed with ethanol, and vacuum dried at 80°C to obtain sulfonation Aluminum trichloride supported on polystyrene microspheres.
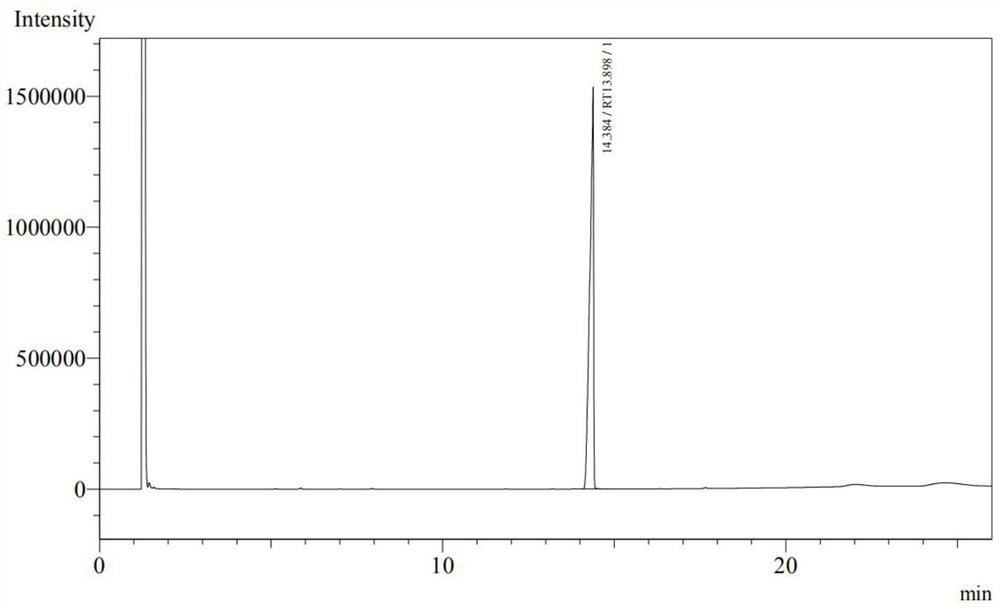
[0059] (2) adding stearyl alcohol to maleic anhydride in a molar ratio of 1:1.1 into a cyclohexane solvent, heating to 90° C., stirring and refluxing for 5 hours to obtain monostearyl maleate. The yield was 90.7%. The product was detected according to the Chinese Pharmacopoeia CP2020 version. The peak time of monostear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com