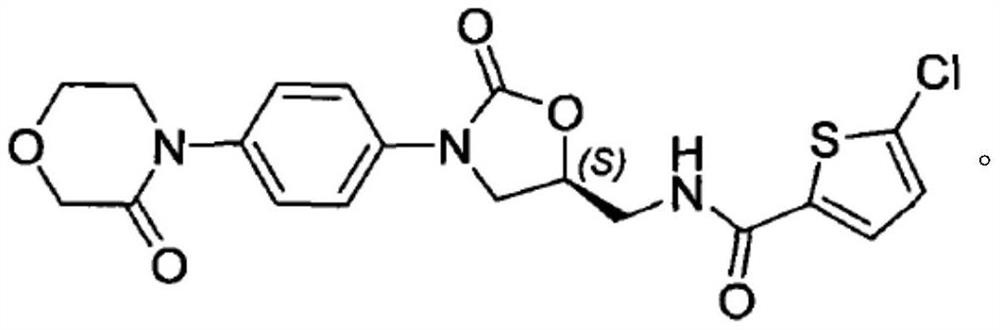
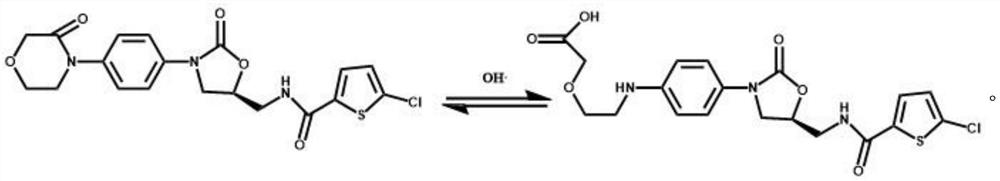
Rivaroxaban pharmaceutical composition
A technology of rivaroxaban and its composition, which is applied in the field of medicine, can solve the problems of individual differences in vivo and in vitro, and achieve the effect of avoiding the use of surfactants and improving dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
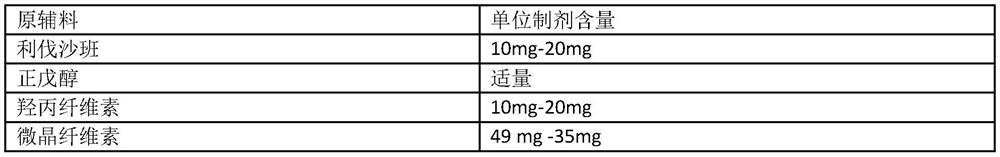
[0105] Example 1 Preparation of rivaroxaban film-coated tablets with a specification of 10 mg (unit: g)
[0106] Raw materials Dosage Rivaroxaban 10g n-pentanol Appropriate amount Hypromellose 10g microcrystalline cellulose 49g Mannitol 25g Croscarmellose Sodium 5g Sodium stearyl fumarate 1g Co-made 1000 pieces Opadry(R)II 3%-5% weight gain
[0107] Preparation Process:
[0108] 1) Take the rivaroxaban raw material, dissolve it with an appropriate amount of n-amyl alcohol, add hyprolose, and dissolve to obtain a drug-containing solution;
[0109] 2) Take the prescription amount of microcrystalline cellulose, mannitol, and croscarmellose sodium and put them in a fluidized bed, and enter the air to obtain a boiling material;
[0110] 3) Spray the drug-containing solution obtained in step 1) into the boiling material in step 2) in the form of a bottom spray, and remove the solvent through the action of hot a...
Embodiment 2
[0114] Example 2 Preparation of Rivaroxaban Film-Coated Tablets with Specifications of 15 mg (unit: g)
[0115] Raw materials Dosage Rivaroxaban 15g n-pentanol Appropriate amount Hypromellose 15g microcrystalline cellulose 42g Mannitol 21g Croscarmellose Sodium 5g Sodium stearyl fumarate 2g Co-made 1000 pieces Opadry(R)II 3%-5% weight gain
[0116] Preparation Process:
[0117] 1) Take the rivaroxaban raw material, dissolve it with an appropriate amount of n-amyl alcohol, add hyprolose, and dissolve to obtain a drug-containing solution;
[0118] 2) Take the prescription amount of microcrystalline cellulose, mannitol, and croscarmellose sodium and put them in a fluidized bed, and enter the air to obtain a boiling material;
[0119] 3) Spray the drug-containing solution obtained in step 1) into the boiling material in step 2) in the form of a bottom spray, and remove the solvent through the action of hot ai...
Embodiment 3
[0123] Example 3 Preparation of rivaroxaban film-coated tablets with a specification of 20 mg (unit: g)
[0124] Raw materials Dosage Rivaroxaban 20g n-pentanol Appropriate amount Hypromellose 20g microcrystalline cellulose 35g Mannitol 18g Croscarmellose Sodium 5g Sodium stearyl fumarate 2g Co-made 1000 pieces Opadry(R)II 3%-5% weight gain
[0125] Preparation Process:
[0126] 1) Take the rivaroxaban raw material, dissolve it with an appropriate amount of n-amyl alcohol, add hyprolose, and dissolve to obtain a drug-containing solution;
[0127] 2) Take the prescription amount of microcrystalline cellulose, mannitol, and croscarmellose sodium and put them in a fluidized bed, and enter the air to obtain a boiling material;
[0128] 3) Spray the drug-containing solution obtained in step 1) into the boiling material in step 2) in the form of a bottom spray, and remove the solvent through the action of hot a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com