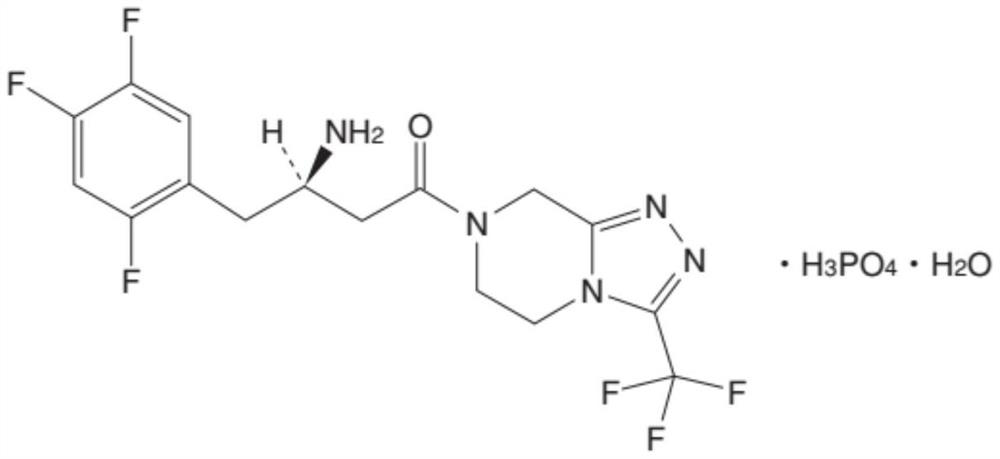
Sitagliptin pharmaceutical composition and preparation process thereof
A composition and drug technology, applied in drug combination, drug delivery, drug formulation and other directions, can solve the problems of weight difference, poor product stability, sticking and punching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3 and comparative Embodiment 1-3
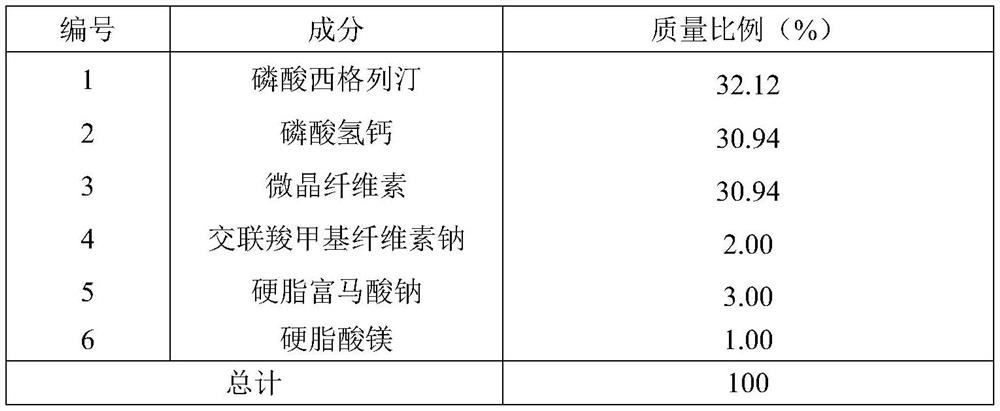
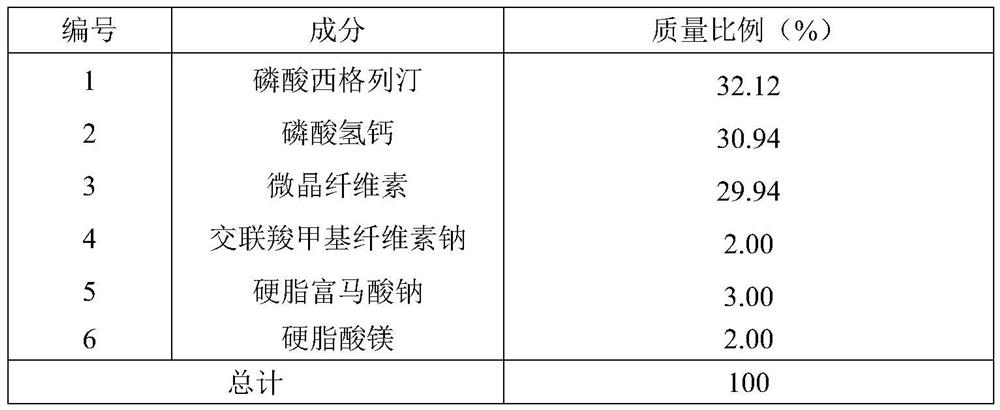
[0043] Preparation of Sitagliptin Phosphate Tablets, the material composition is shown in Table 1-2
[0044] Table 1 embodiment 1-3 and the material composition ratio of comparative example 1-2
[0045]
[0046] The material composition ratio of table 2 comparative example 3
[0047]
[0048] The particle size (laser particle size analyzer detection) of active ingredient in the concrete embodiment of table 3
[0049]
[0050] The coating parameter that table 4 specific embodiment uses
[0051]
Embodiment 1-3
[0052] Embodiment 1-3 and comparative example 1-3 processing steps are:
[0053] 1. Sitagliptin phosphate is mixed with microcrystalline cellulose, calcium hydrogen phosphate, and croscarmellose sodium;
[0054] 2. The mixture is then sized by a rotary granulator. The sizes of the sieves of the granulator used in Examples 1 to 3 are 0.8mm, 1.0mm, and 1.2mm, respectively.
[0055] 3. Add the sieved sodium stearyl fumarate and magnesium stearate and continue mixing.
[0056] 4. Tablet pressing.
[0057] 5. Carry out coating according to the coating parameter of table 4, coating material is selected from series.
[0058] The product quality was tested, and the results are shown in Table 5.
[0059] Table 5 Test results of sitagliptin phosphate tablets
[0060]
[0061] Analysis of Table 1-5 results:
[0062] According to embodiment 1-3, the product placed under accelerated conditions for 6 months is surprisingly found that the product stability has a significantly bette...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Graininess | aaaaa | aaaaa |
| Graininess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com