Preparation method of sitagliptin phosphate intermediate
A technology for sitagliptin phosphate and intermediates, applied in the field of preparation of sitagliptin phosphate intermediates, can solve the problems of low solvent recovery rate, unfavorable environmental protection, influence and the like, achieve high purity, improve yield and purity, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
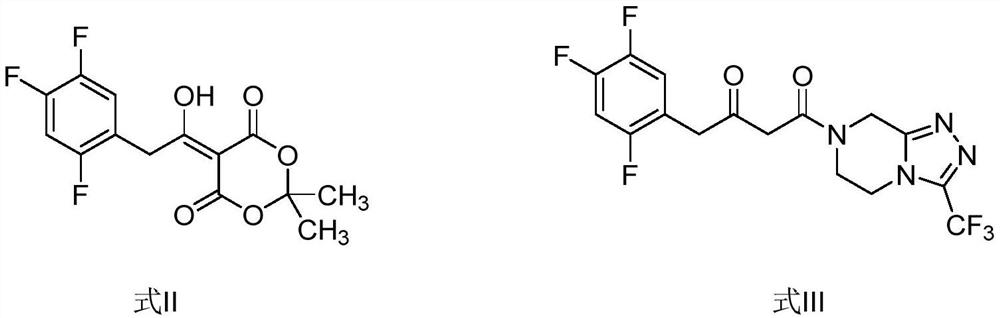
[0028] The preparation of compound shown in embodiment 1 formula II
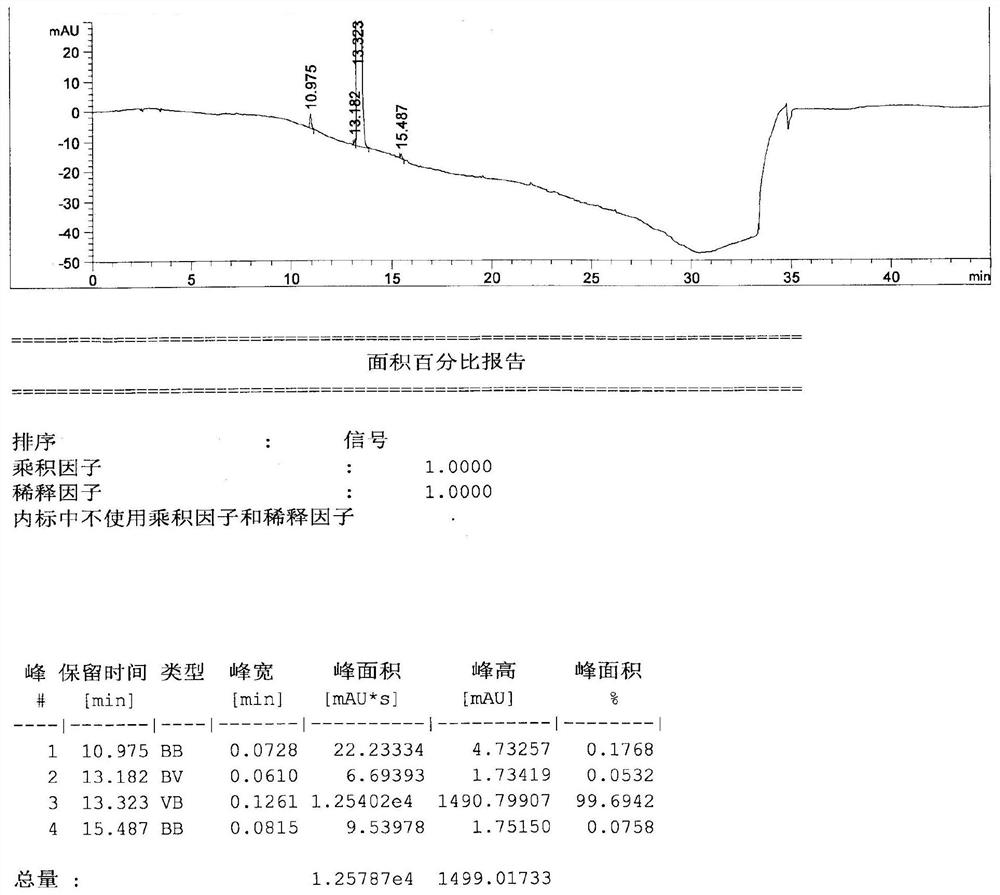
[0029] Add 2.50kg of acetonitrile, 1.00kg of trifluorophenylacetic acid, 0.85kg of Michaelis acid, 50.00g of 4-dimethylaminopyridine in a 10L reaction kettle, and then dropwise add 1.50kg of N,N-diisopropylethylamine and 0.70kg of pivaloyl chloride After the addition, the reaction was carried out at 45°C for 6.0h. After the reaction was completed, the solvent was recovered under reduced pressure. After recovering to dryness, add 10.00kg of 75% ethanol, add 1.00kg of hydrochloric acid under stirring, stir and crystallize at 10°C, stir for 5.0h and then filter, wash the filter cake with 1.00kg of 75% ethanol, and vacuum dry at 45°C for 20.0h to obtain Sigiryl Phosphate Gliptin intermediate II, yield 93%, purity 99.7%.
Embodiment 2
[0030] Preparation of compound shown in embodiment 2 formula III
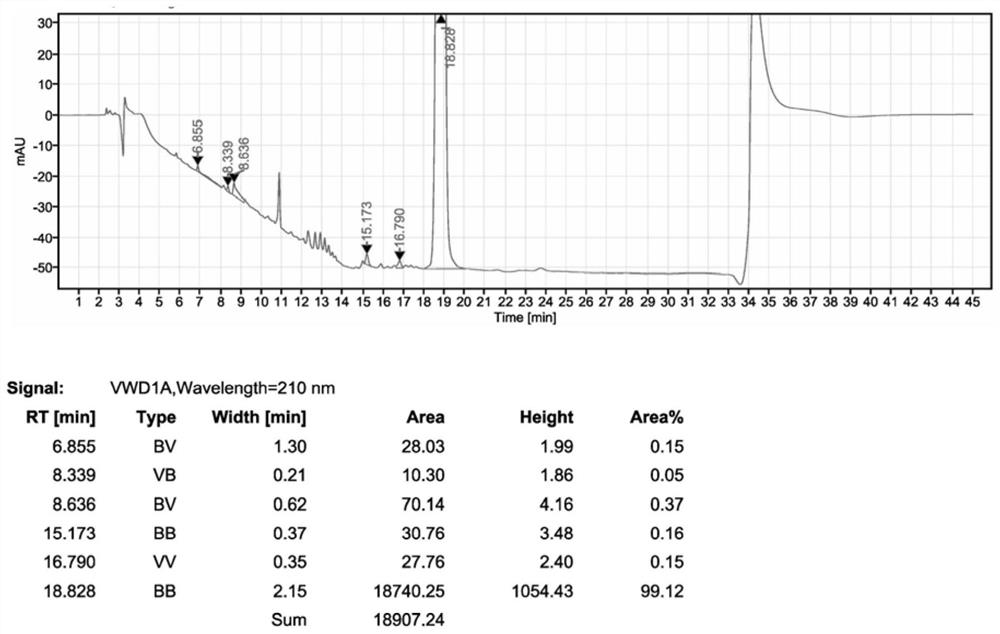
[0031] Drop into 5.00kg acetonitrile, 1.50kg sitagliptin phosphate intermediate II, 0.80kg N,N-diisopropylethylamine, 1.20kg pyrazine hydrochloride in 10L reactor, add 0.20kg trifluoroacetic acid, heat up to React at 75°C for 6.0h. After the reaction is completed, the solvent is recovered under reduced pressure. After the recovery under reduced pressure, 6.00kg of 75% ethanol is added to stir and crystallize at 10°C. After stirring for 5.0h, the filter cake is washed with 2.00kg of 75% ethanol. Dry in a vacuum oven at 45°C to obtain sitagliptin phosphate intermediate III with a yield of 93% and a purity of 99.1%.
Embodiment 3
[0032] Preparation of compound shown in embodiment 3 formula II
[0033] Add 25g of acetonitrile, 10g of trifluorophenylacetic acid, 8.5g of Michaelis acid, 0.5g of 4-dimethylaminopyridine into a 100mL flask, and add 15g of N,N-diisopropylethylamine and 7g of pivaloyl chloride dropwise successively. The reaction was carried out at 45°C for 6 hours. After the reaction was completed, the solvent was recovered under reduced pressure. After recovering to dryness, add 100g of 30% ethanol, add 10g of hydrochloric acid under stirring, stir and crystallize at 10°C, stir for 5.0h and then filter, wash the filter cake with 10g of 30% ethanol, and vacuum dry at 45°C for 20h to obtain sitagliptin phosphate intermediate Body II, yield 95%, purity 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com