Sitagliptin phosphate crystal and preparation method and application thereof
A technology of sitagliptin phosphate and phosphoric acid aqueous solution, which is applied in the field of pharmaceutical chemical crystallization, can solve the problems of different drug dissolution rate and bioavailability, influence on drug absorption and utilization, and difference in drug solubility, so as to reduce drug load and improve Drug efficacy, the effect of reducing production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Preparation of Sitagliptin Phosphate Monohydrate Crystals
[0070] At room temperature, 10.175 g of sitagliptin, 43.5 mL of isopropanol, and 11.25 mL of water were added to the reactor, and then 5 mL of 45% phosphoric acid aqueous solution was added dropwise; the temperature was raised to 75 °C, and dissolved into a transparent liquid; cooled to 70 °C, The reaction was stirred for 3 hours. Then, the solution was stirred and cooled to 60°C at a cooling rate of 8°C per hour; then it was left to stand, and during the standing process, the solution was cooled to 44°C at a cooling rate of 8°C per hour; then cooled to 21°C with stirring in 30 minutes; White solid. Within 30 minutes, 32 mL of isopropanol was added dropwise, and then stirred for 15 minutes; suction filtration, washed twice with 40.7 mL of isopropanol-water (V / V=6:1) system; after vacuum degree -0.08MPa, 25 It was dried at °C for 3 hours, and dried at 40 °C for 3 hours to obtain 12.59 g of a white...
Embodiment 2
[0076] Example 2: Preparation of Sitagliptin Phosphate Monohydrate Crystals
[0077] At room temperature, 12.21 g of sitagliptin, 52 mL of isopropanol, and 13.5 mL of water were added to the reactor, and then 6 mL of a 45% volumetric phosphoric acid aqueous solution was added dropwise; the temperature was raised to 75 °C, and dissolved into a transparent liquid; then cooled to 65 °C, and the reaction was stirred for 3 hours. Then cool down to 25°C with stirring at a cooling rate of 8°C per hour; within 30 minutes, add 38 mL of isopropanol dropwise, then stir for 30 minutes, filter with suction, and use isopropanol-water (V / V=6:1) 56 mL of the system was washed twice. Dry at -0.08MPa and 35°C for 5 hours to obtain 15.17g of white crystalline solids. After detection, HPLC (%): 99.85%; moisture: 3.64%; molar yield: 96.7%.
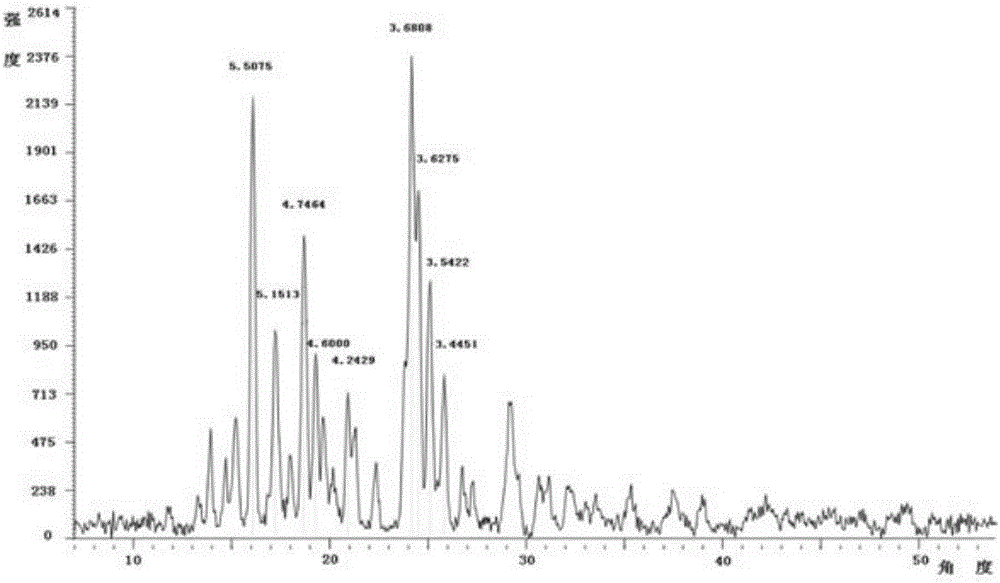
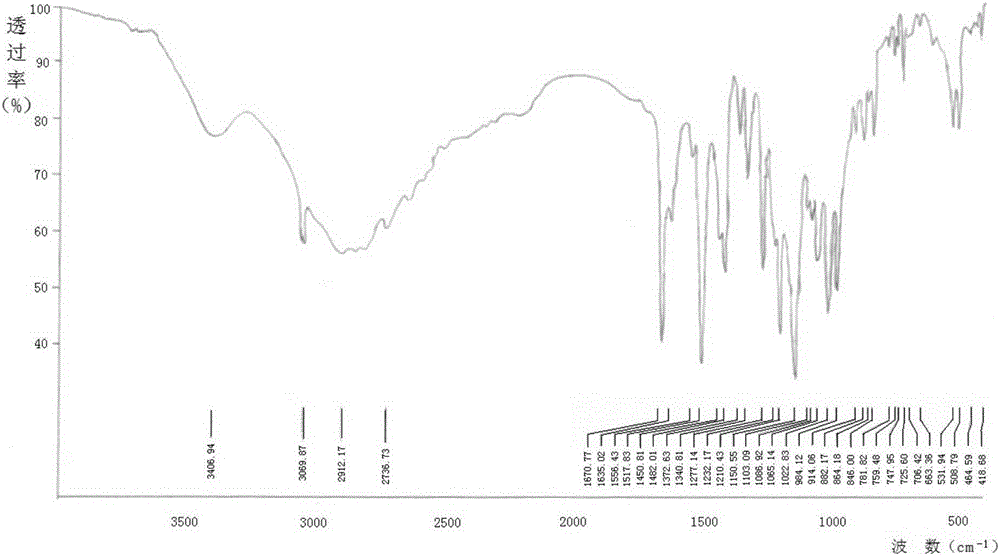
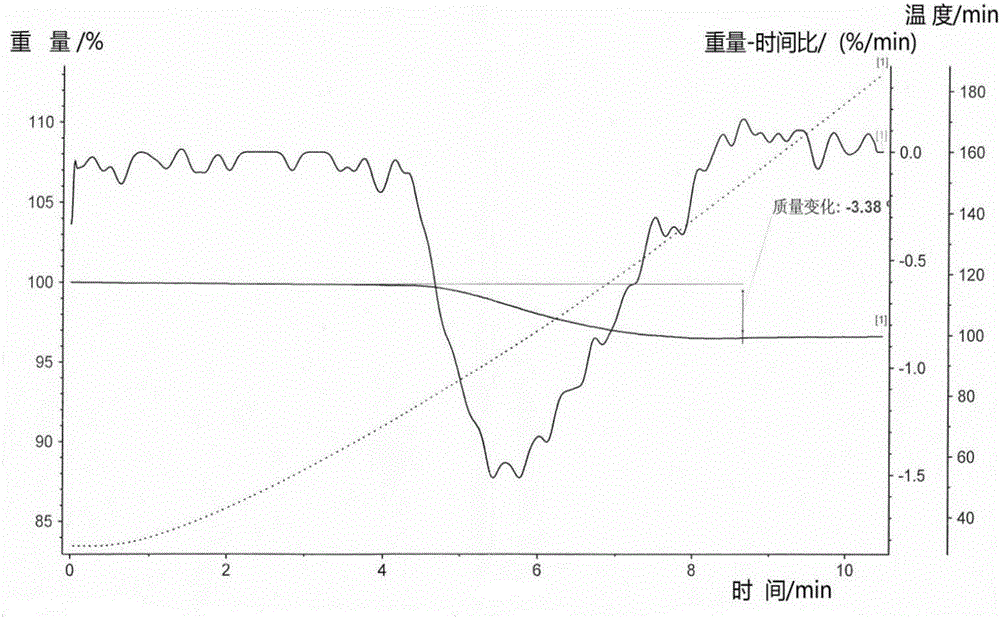
[0078] The X-ray diffraction pattern, infrared spectrum and differential scanning calorimetry (DSC) curve of the sitagliptin phosphate monohydrate crystal...
Embodiment 3
[0079] Example 3: Preparation of Sitagliptin Phosphate Monohydrate Crystals
[0080] At room temperature, 8.14 g of sitagliptin, 16.3 mL of isopropanol, and 7.3 mL of water were added to the reactor, and then 1.73 mL of 85% phosphoric acid aqueous solution was added dropwise; the temperature was raised to 80 °C, and dissolved into a transparent liquid; cooled to The reaction was stirred for 2 hours at 70°C. After that, it was stirred and cooled to 54°C at a cooling rate of 8°C per hour, and then cooled to 22°C with a cooling rate of 16°C per hour, and a large amount of white solids were precipitated. Within 40 minutes, 57 mL of isopropanol was added dropwise, and then stirred for 15 minutes; filtered with suction, washed twice with 40.7 mL of isopropanol-water (V / V=11:1) system; After drying at °C for 5.5 hours, 10.22 g of a white crystalline solid was obtained. After detection, HPLC (%): 99.91%; moisture: 3.54%; molar yield: 97.7%.
[0081] The X-ray diffraction pattern, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com