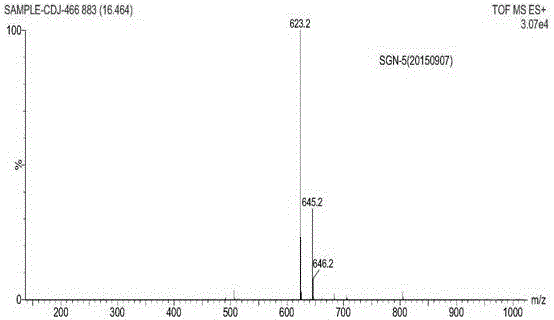
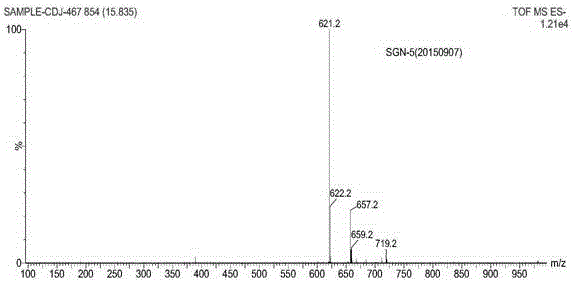
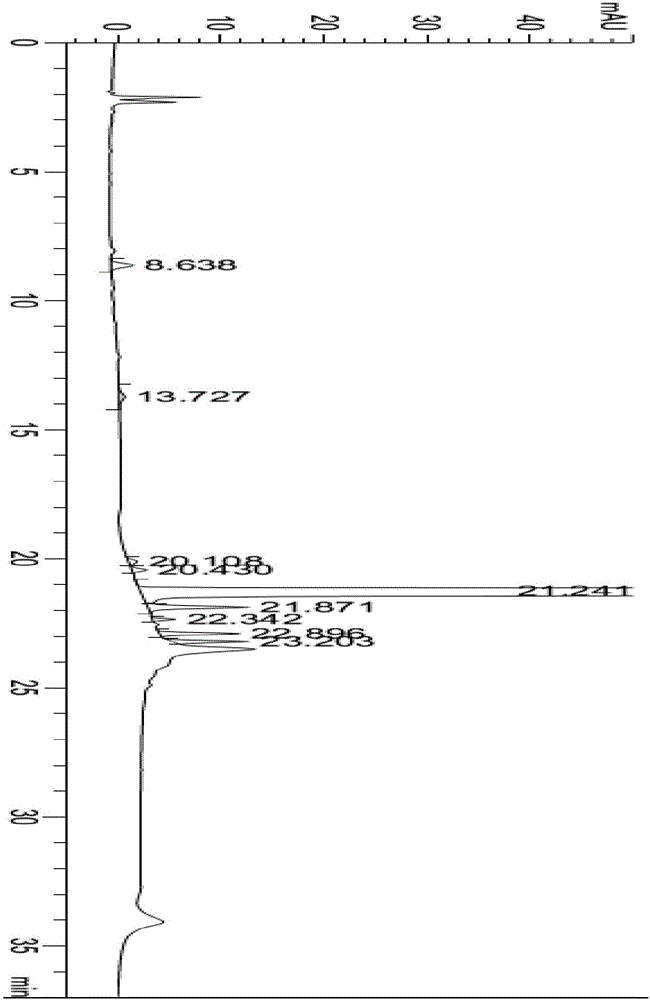
Sitagliptin impurity and preparation and detection method thereof
A technology for sitagliptin impurities and impurities, which is applied in the field of sitagliptin impurities and preparation, and achieves the effects of mild reaction conditions, guaranteed safety and reliability, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 10g of 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-trifluoro Methyl-1,2,4-triazolo[4,3-a]pyrazine, 8.2g (R)-3-(tert-butoxycarbonyl)amino-4-(2,4,5-trifluorobenzene Base) butyric acid and 200mL methanol were added to a 500mL reaction flask, stirred and dissolved, then cooled to -10~-5°C, and 6.8g 4-(4,6-dimethoxytriazin-2-yl)-4-methanol was added Morpholine hydrochloride, 4.9g of triethylamine were reacted for 3 hours. Put the reaction bottle at room temperature, add 150mL of water, stir for 45min, filter, and dry the filter cake to obtain 16.2g of compound II.
[0040] 16.2 g of compound II obtained above, 200 mL of dichloromethane, and 15 g of trifluoroacetic acid were added into a 500 mL reaction flask and heated to reflux for reaction, and monitored by TLC until the reaction of the raw materials was completed. Lower the reaction temperature to room temperature, add 200% water to the reaction bottle, adjust the pH to about 8-9 with 2mol...
Embodiment 2
[0042] 10g of 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-trifluoro Methyl-1,2,4-triazolo[4,3-a]pyrazine, 9.7g (R)-3-(tert-butoxycarbonyl)amino-4-(2,4,5-trifluorobenzene Base) butyric acid, 200mL acetonitrile were added to a 500mL reaction flask, stirred and dissolved, then cooled to -8°C, and 7.3g of 4-(4,6-dimethoxytriazin-2-yl)-4-methylmorpholine salt was added Acetate, 5.6g triethylamine back reaction 2.5 hours. Put the reaction bottle at room temperature, add 150mL of water, stir for 45min, filter, and dry the filter cake to obtain 16.4g of compound II.
[0043] Add 16.4 g of compound II obtained above, 180 mL of toluene, and 6 g of hydrochloric acid into a 500 mL reaction flask and heat to reflux for reaction. TLC monitors until the reaction of the raw materials is complete. Lower the reaction temperature to room temperature, add 180% water to the reaction bottle, adjust the pH to about 8-9 with 2mol / L sodium hydroxide, extract, extract the...
Embodiment 3
[0045] 10g of 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-trifluoro Methyl-1,2,4-triazolo[4,3-a]pyrazine, 10.2g (R)-3-(tert-butoxycarbonyl)amino-4-(2,4,5-trifluorobenzene base) butyric acid and 230mL acetone were added to a 500mL reaction flask, stirred and dissolved, then cooled to -5°C, and 4.8g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride, 3.6g Ethylenediamine post reaction for 3.5 hours. Put the reaction bottle at room temperature, add 170mL of water, stir for 45min, filter, and dry the filter cake to obtain 16.1g of compound II.
[0046] Add 16.1 g of Compound II obtained above, 180 mL of toluene, and 10.3 g of formic acid into a 500 mL reaction flask and heat to reflux for reaction. TLC monitors until the reaction of the raw materials is complete. Lower the reaction temperature to room temperature, add 180% water to the reaction bottle, adjust the pH to about 8-9 with 2mol / L sodium hydroxide, extract, extract the dichlorom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com