Compositions and methods of treating middle-of-the night insomnia
a technology of compositions and methods, applied in the direction of drug compositions, biocides, heterocyclic compound active ingredients, etc., can solve the problems of unsuitable hypnotic medications for treating motn insomnia, unnecessary medication and overmedication of persons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Low Dose Zolpidem Lozenge Compositions
[0193] Individuals suffering from middle-of-the-night insomnia are given lozenges containing 0 mg, 1.0 mg, 1.75 mg, or 3.5 mg zolpidem for sublingual delivery that are prepared according to the formulations set forth in Table 3.
TABLE 3Low dose zolpidem lozenge formulations.Quantity (mg / lozenge)StrengthComponentPlacebo1.0 mg1.75 mg1.75 mg3.5 mgZolpidem hemitartrate01.01.751.753.5Pharmaburst ™ B214314270141.25139.5Consisting of:mannitolsorbitolcrospovidonesilicon dioxideCroscarmellose sodium101051010Sodium carbonate17178.51717Sodium bicarbonate232311.52323Natural and artificial6.56.53.256.56.5spearmint FONA#913.004Silicon dioxide5.55.52.755.55.5Sucralose1.51.50.751.51.5Magnesium stearate3.53.51.753.53.5Total lozenge weight210210105210210
[0194] The individuals self-administer one lozenge of the above formulations when their sleep is interrupted and they have at least 2 hours of sleep time remaining. Upon awakening, the individuals provide a subj...
example 2
Pharmacokinetic and Pharmacodynamic Investigation of Low Dose Zolpidem Lozenge Compositions
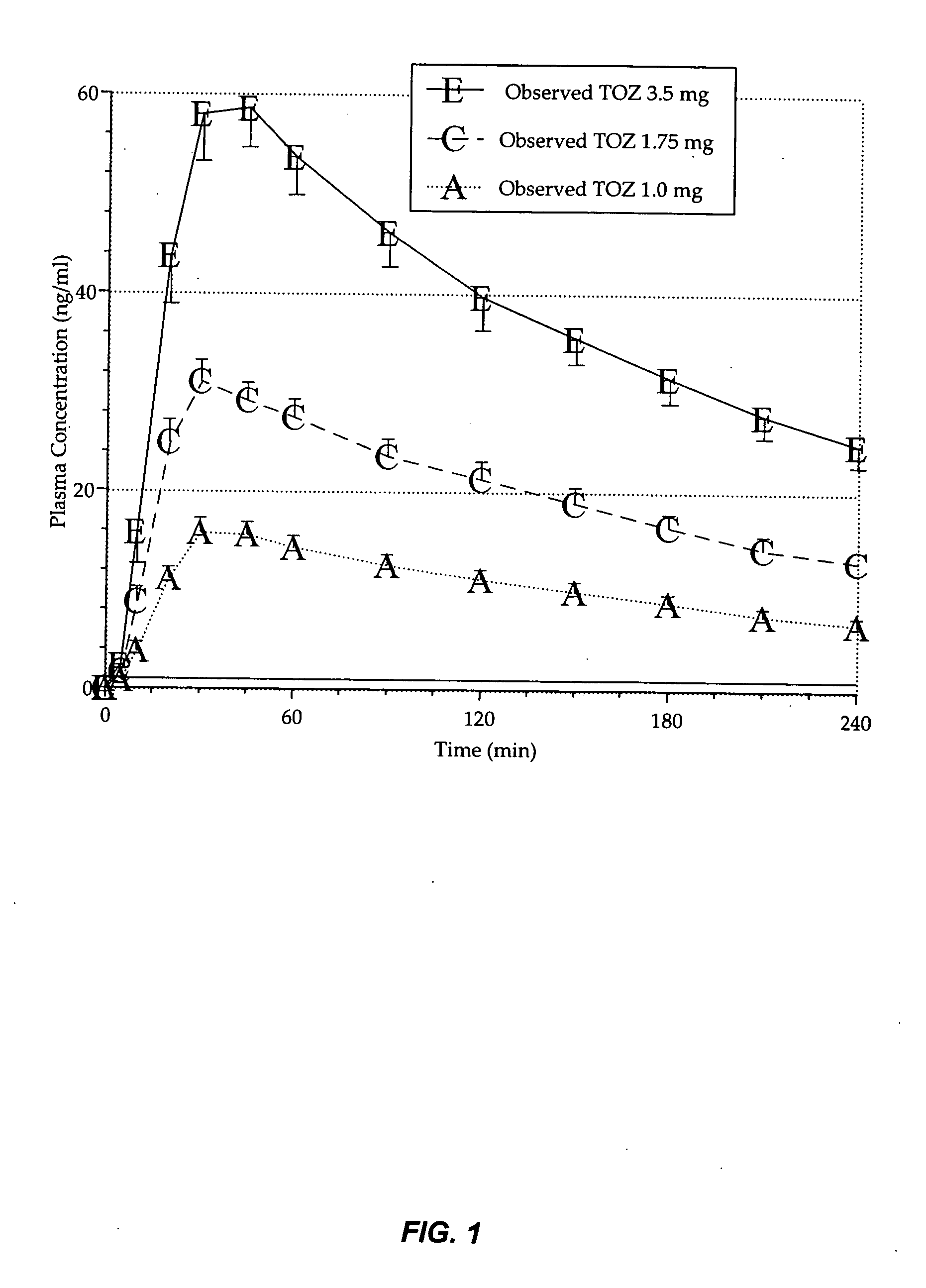
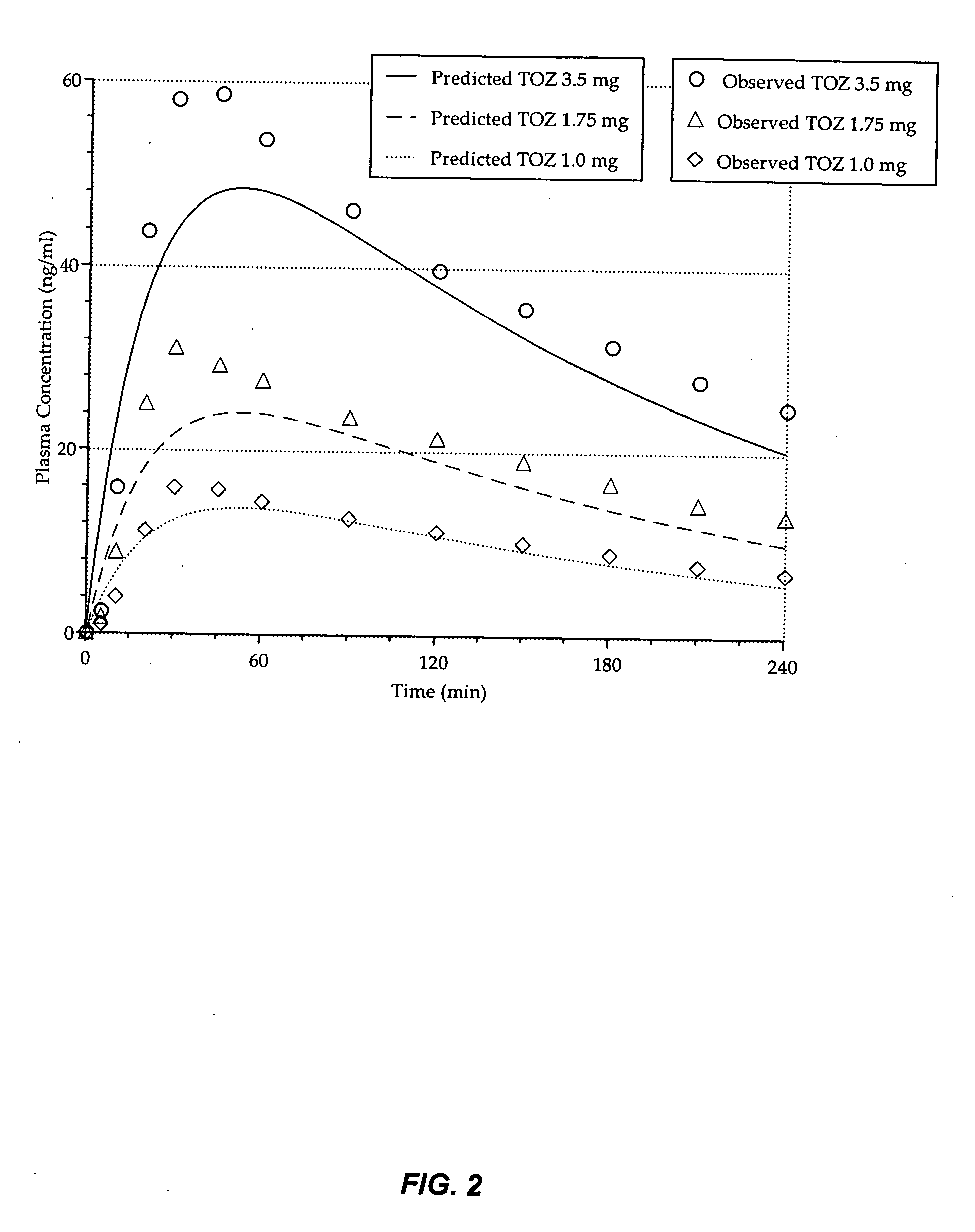
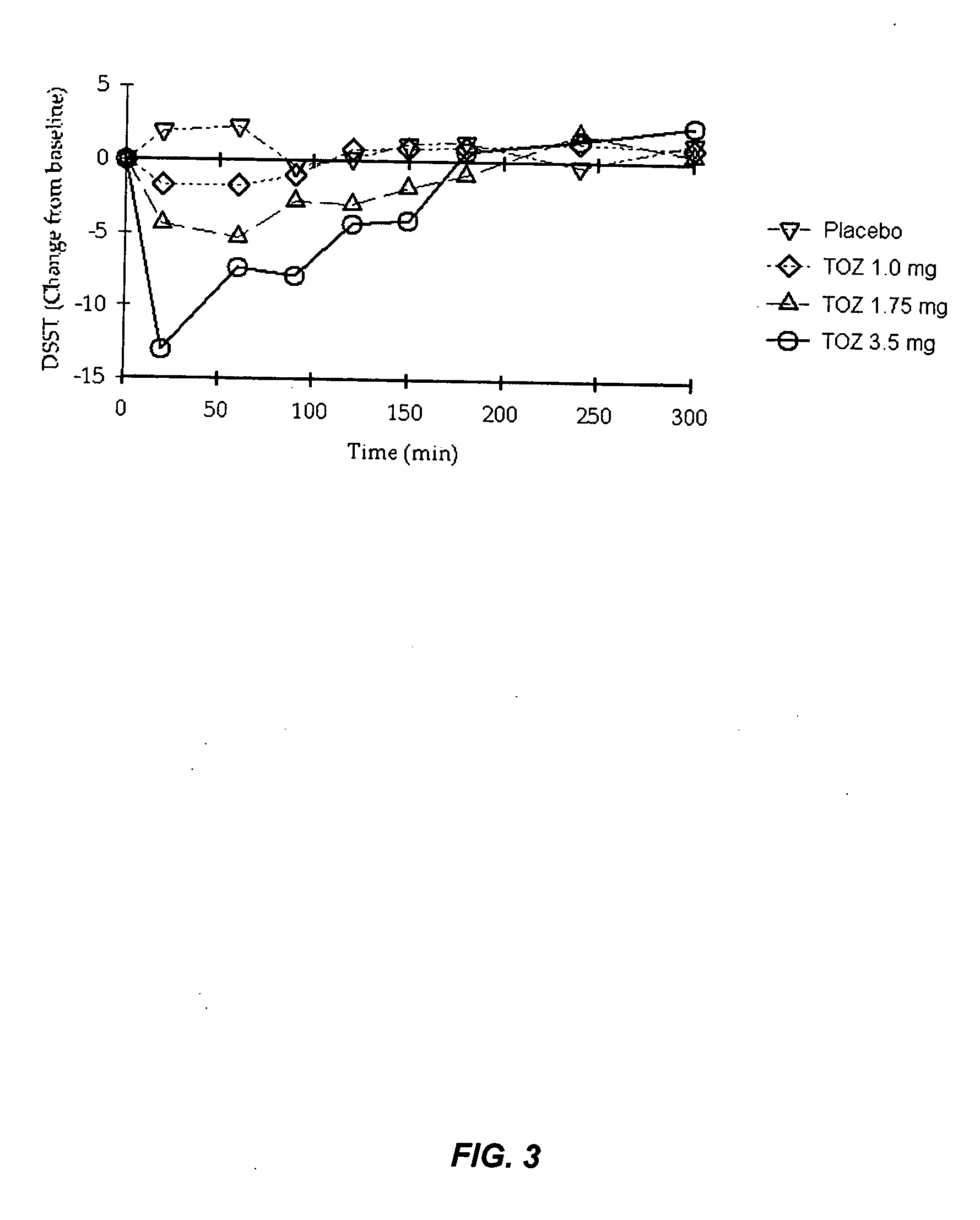
[0196] This example provides an evaluation of the daytime dose-dependent pharmacokinetic and pharmacodynamic effects of the 1.0 mg, 1.75 mg, and 3.5 mg zolpidem lozenges described in Table 3 above.
Summary
[0197] Currently, no medications are available to be used on apro re nata basis for patients who have middle-of-the-night (MOTN) awakening and who have difficulty falling back asleep. An appropriate therapeutic agent for such insomnia would enable patients to return to sleep rapidly and wake up in the morning without residual effects. This study illustrates, inter alia, that the low dose zolpidem lozenges of the present invention enhance rapid systemic delivery of zolpidem without affecting other pharmacokinetic parameters.
[0198] Healthy adults (n=24; mean age=37.6 yrs) participated in this double-blind, placebo-controlled, 4-way crossover study of 2 consecutive days of morning dosing wit...
example 3
Low Dose Zolpidem Tablet Composition
[0228] An immediate release peroral (PO) tablet containing a low dose of zolpidem can be prepared according to the formulation set forth in Table 5.
TABLE 5Low dose zolpidem tablet formulation.ComponentQuantity (mg)Zolpidem Hemitartrate3.5Povidone K29 / 3215.0Sodium Starch Glycolate (SSG)7.5Starch 150015.0Lactose Fast Flow82.0Prosolv SMCC 9065.5Sodium bicarbonate40Magnesium Stearate1.5Total230
Manufacturing Process
[0229] Dispensing: Screen the zolpidem hemitartrate and excipients through screen #30. Dispense the required quantities of each ingredient.
[0230] Blending: [0231] 1. Transfer the zolpidem hemitartrate and Povidone K 29 / 32 to a V-Shell blender and blend for 2 min. [0232] 2. Add SSG and Starch 1500 to Step 1 and blend for another 2 min. [0233] 3. Add Lactose Fast Flow and Prosolv SMCC 90 to Step 2 and blend for another 10 min. [0234] 4. Mix an equal amount of the blend from Step 3 with magnesium stearate or sodium stearyl fumarate and tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com