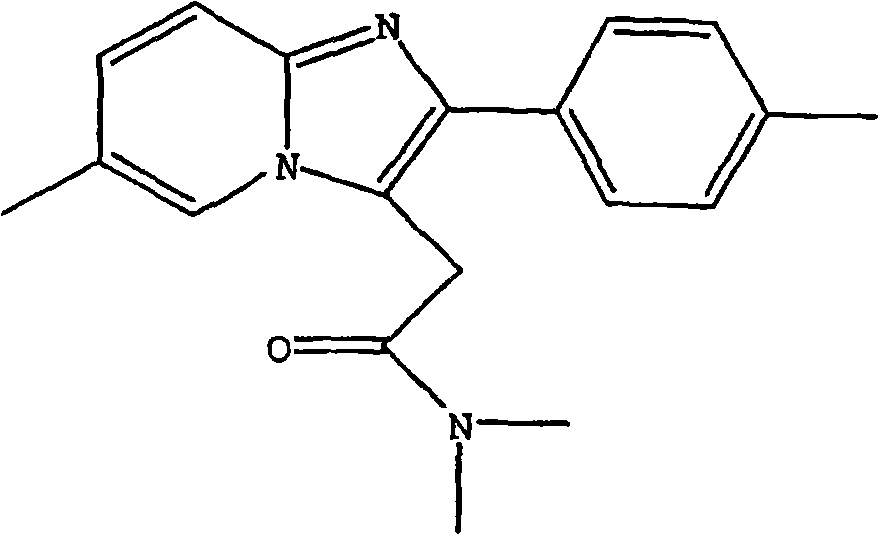
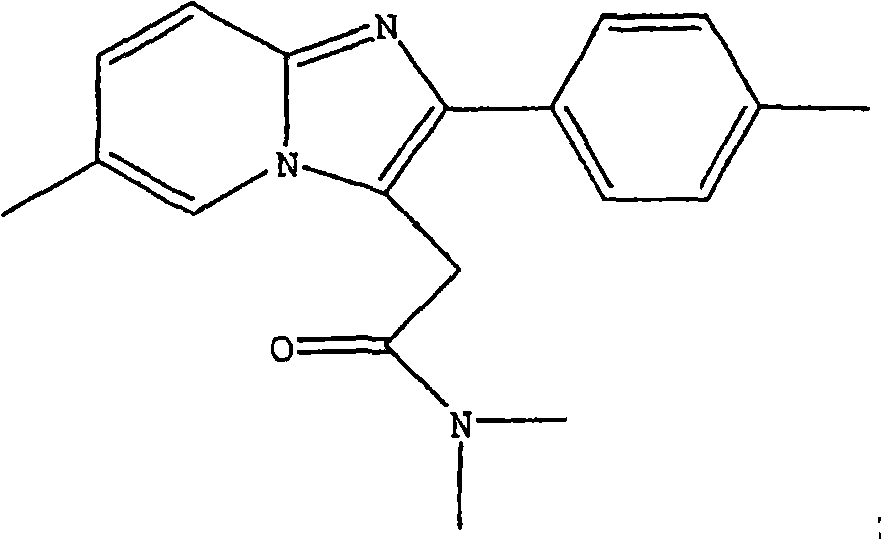
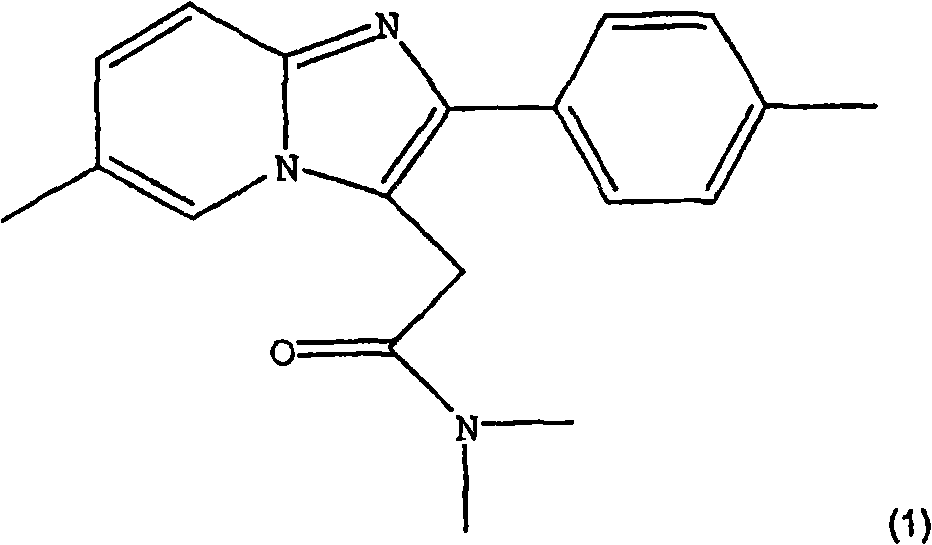
Process for preparing zolpidem hemitartrate and tartrate polymorphs
A technology of hemi-tartrate and tartaric acid, applied in organic chemistry, nervous system diseases, drug combination, etc., can solve the problems of difficult processing of polymorphs and increased production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Selective preparation of polymorph E
[0028]Zolpidem free base (5 g) was dissolved in methanol (36 mL) to give a first solution. L-(+)-tartaric acid (1.2 g) was dissolved in methanol (12 mL) to produce a second solution. The first solution and the second solution were mixed in a flask to obtain a feed solution, which was stirred at 65° C. for 30 minutes. Water (50 mL) was added to the sample solution. The sample solution was distilled until 50 mL of distillate was distilled from the sample solution. Distillation resulted in a sample solution containing less than 30% residual methanol. The remaining sample solution was cooled to ambient temperature with stirring until a solid product precipitated from the sample solution. The solution was then rotary evaporated to dryness at a pressure of 100 mb and a temperature of 40°C to obtain a dry powder. The dry powder was removed from the flask by adding 100 mL of water. Scrape the flask to remove as much powder...
Embodiment 2
[0030] Example 2. Selective preparation of polymorph E
[0031] Zolpidem free base (5 g) was dissolved in methanol (36 mL) to give a first solution. L-(+)-tartaric acid (1.2 g) was dissolved in methanol (12 mL) to produce a second solution. The first solution and the second solution were combined to produce a sample solution, which was stirred at 65°C for 30 minutes. The sample solution was distilled at 75°C until the effluent distilled from the sample solution was 15 mL. Water (25 mL) was then added to the sample solution and further distilled until the vapor temperature reached 94°C. At this point, approximately 30 mL was distilled again such that the final volume of distillate collected was 45 mL. The remaining sample solution was cooled to ambient temperature with stirring until a solid product precipitated from the sample solution. The zolpidem hemitartrate suspension was filtered through a vacuum funnel and the powder collected in the filter. Save the filtrate for l...
Embodiment 3
[0034] Example 3. Selective preparation of polymorph H
[0035] Zolpidem base (1 g) and L-(+)-tartaric acid (0.24 g) were dissolved in ethanol (10 mL). The solution was stirred at 60°C until all solids had dissolved. The solution was cooled to ambient temperature and a solid precipitated and crystallized. The suspension was filtered and the solid was dried under vacuum for 4 hours to give 1.0 g of powder (yield 81%). An isolated powder was prepared for pXRD analysis, which indicated that the solid contained polymorph H.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com