Timing pulsed release micro-pill of zolpidem salt
A zolpidem tartrate and micropellet technology, which is applied in pharmaceutical formula, drug combination, drug delivery, etc., can solve the problems of long release time, short half-life, short action time, etc., to improve permeability, regulate release, and promote water absorption. chemical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Prescription: g
[0050] Blank core: sucrose and starch 51.6
[0051] Drug Layer: Zolpidem Tartrate 7.5
[0052] Polyvinylpyrrolidone K30 0.5
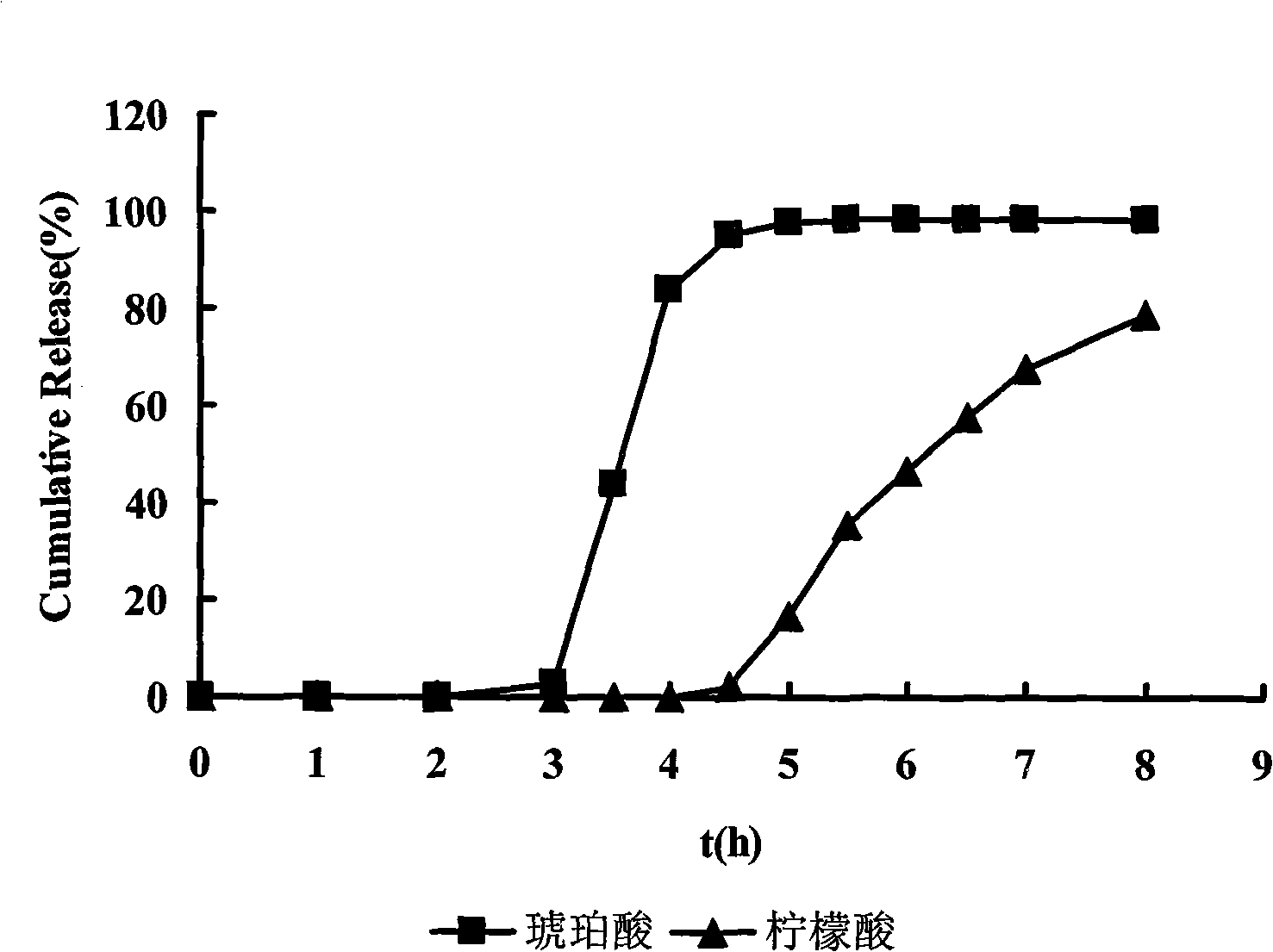
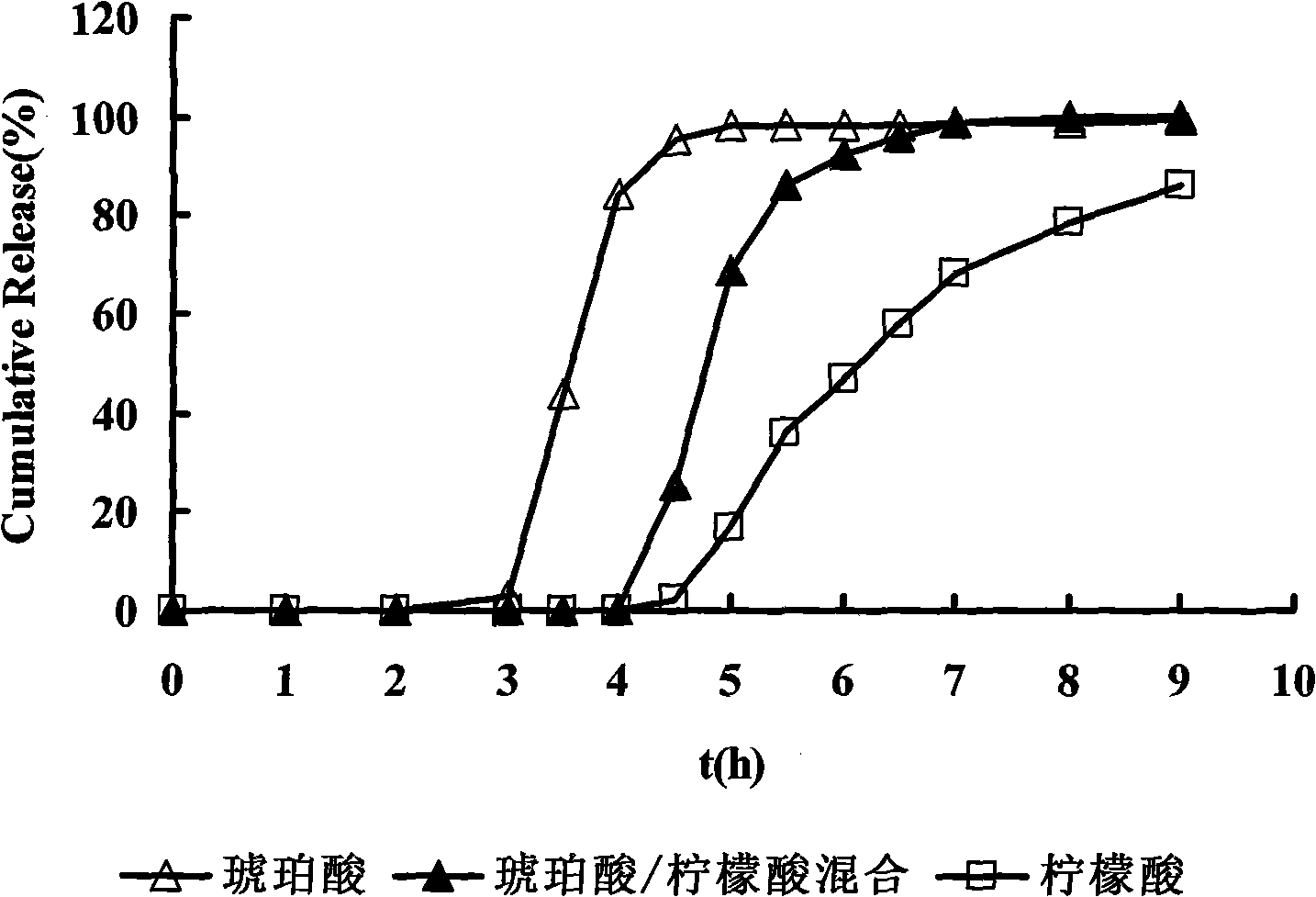
[0053] Organic acid layer: succinic acid 0.8
[0054] Citric acid 0.4
[0055] Polyvinylpyrrolidone K30 0.2
[0056] Lag layer: Eudragit RS30D (according to solid content) 22.9
[0057] Triethyl citrate 4.5
[0058] 1000 mesh superfine talcum powder 11.6
[0059] Preparation:
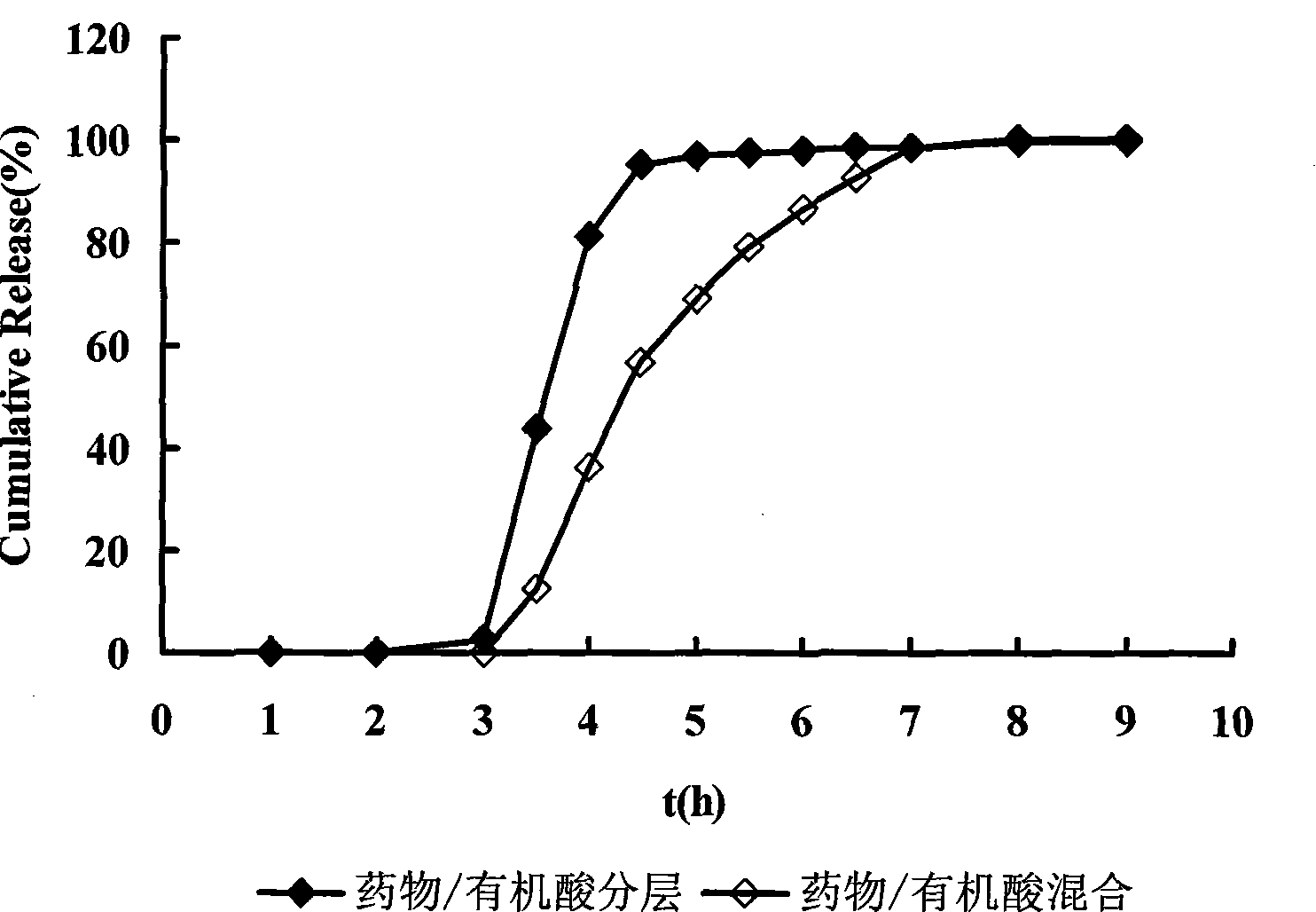
[0060] Dissolve the drug in 2% (g / ml) polyvinylpyrrolidone K30 aqueous solution as the drug application solution. Weigh blank pellet cores and place them in a fluidized bed for preheating for 10 minutes, spray the drug solution onto the blank pellet cores by bottom spraying to prepare drug-containing pellets. The drug-containing pellets were dried in an oven at 60°C for 2 hours for later use. Dissolve succinic acid and citric acid in 2% (g / ml) polyvinylpyrrolidone K30 aqueous ...
Embodiment 2
[0062] Prescription: g
[0063] Blank core: sucrose and starch 51.8
[0064] Drug Layer: Zolpidem Hemi-Tartrate 7.6
[0065] Polyvinylpyrrolidone K30 0.5
[0066] Organic acid layer: succinic acid 0.9
[0067] Citric acid 0.4
[0068] Polyvinylpyrrolidone K30 0.2
[0069] Lag layer: Eudragit RS30D (according to solid content) 22.6
[0070] Triethyl citrate 4.5
[0071] 1000 mesh superfine talcum powder 11.5
[0072] Preparation method: with embodiment 1.
Embodiment 3
[0074] Prescription: g
[0075] Blank core: sucrose and starch 52
[0076] Organic acid layer: succinic acid 2
[0077] Citric acid 0.8
[0078] Polyvinylpyrrolidone K30 0.3
[0079] Drug Layer: Zolpidem Tartrate 7.5
[0080] Polyvinylpyrrolidone K30 0.5
[0081] Lag layer: Eudragit RS30D (according to solid content) 21.7
[0082] Triethyl citrate 4.3
[0083] 1000 mesh superfine talcum powder 10.9
[0084] Preparation:
[0085]Dissolve succinic acid and citric acid in 2% (g / ml) polyvinylpyrrolidone K30 aqueous solution for later use. Weigh the blank pellet core and place it in a fluidized bed to preheat for 10 minutes, spray the organic acid solution onto the blank pellet core in the form of bottom spray to prepare organic acid-containing pellets, and dry them in an oven at 60°C for 2 hours for later use . Zolpidem tartrate was dissolved in 2% (g / ml) polyvinylpyrrolidone K30 aqueous solution, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com