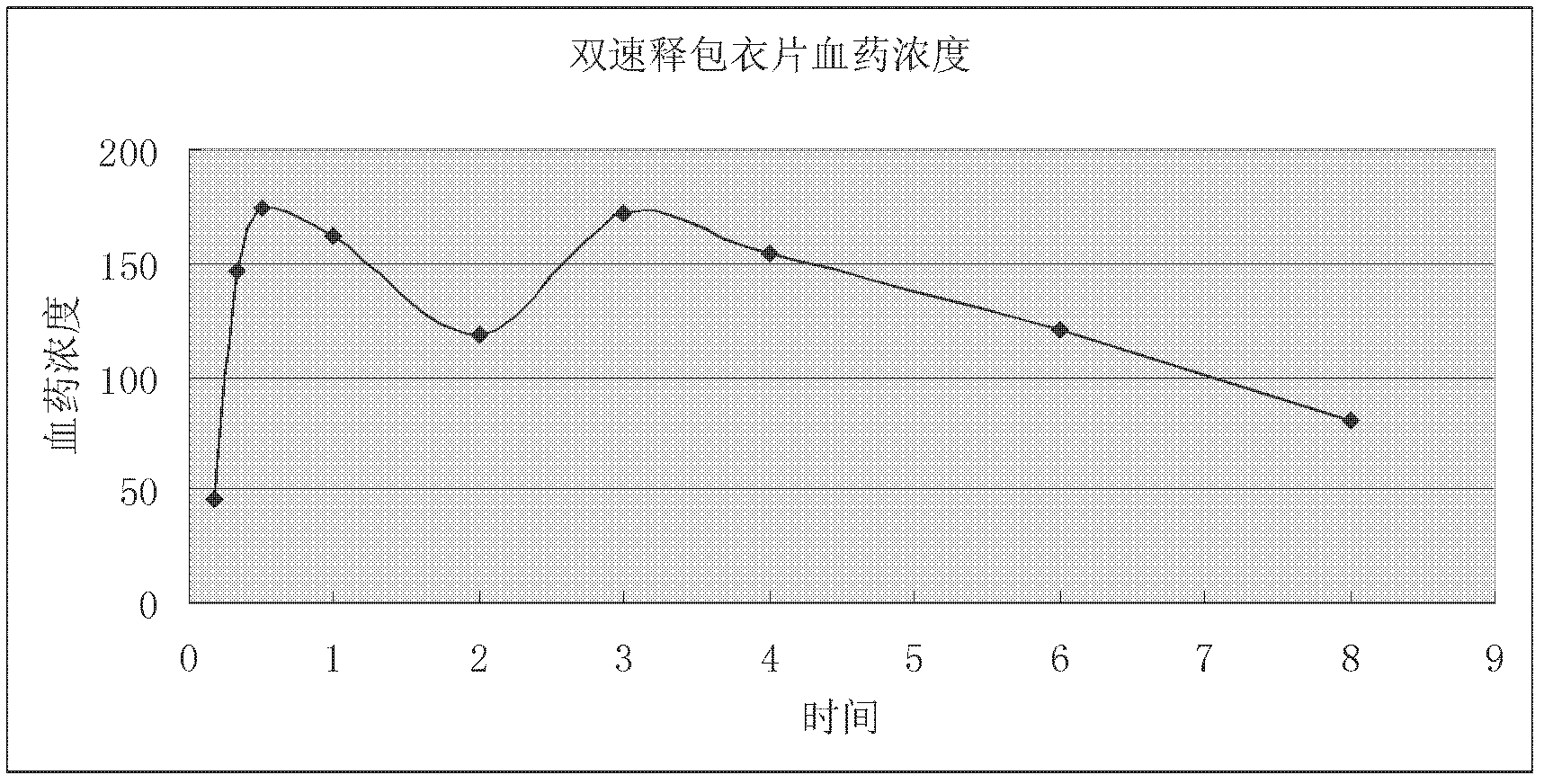
Two-phase release preparation containing zolpidem or salt of zolpidem and preparation method thereof
A technology for zolpidem and immediate-release preparations, which is applied in the field of preparation of biphasic release preparations, can solve the problems such as the inability of drugs to reach an effective drug concentration, difficulty in falling asleep without a therapeutic effect, affecting normal work and life, and the like, so as to improve biological efficiency. Utilization, avoid aging problems, reduce the effect of individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Biphasic Release Tablets Containing 10 mg Zolpidem Tartrate
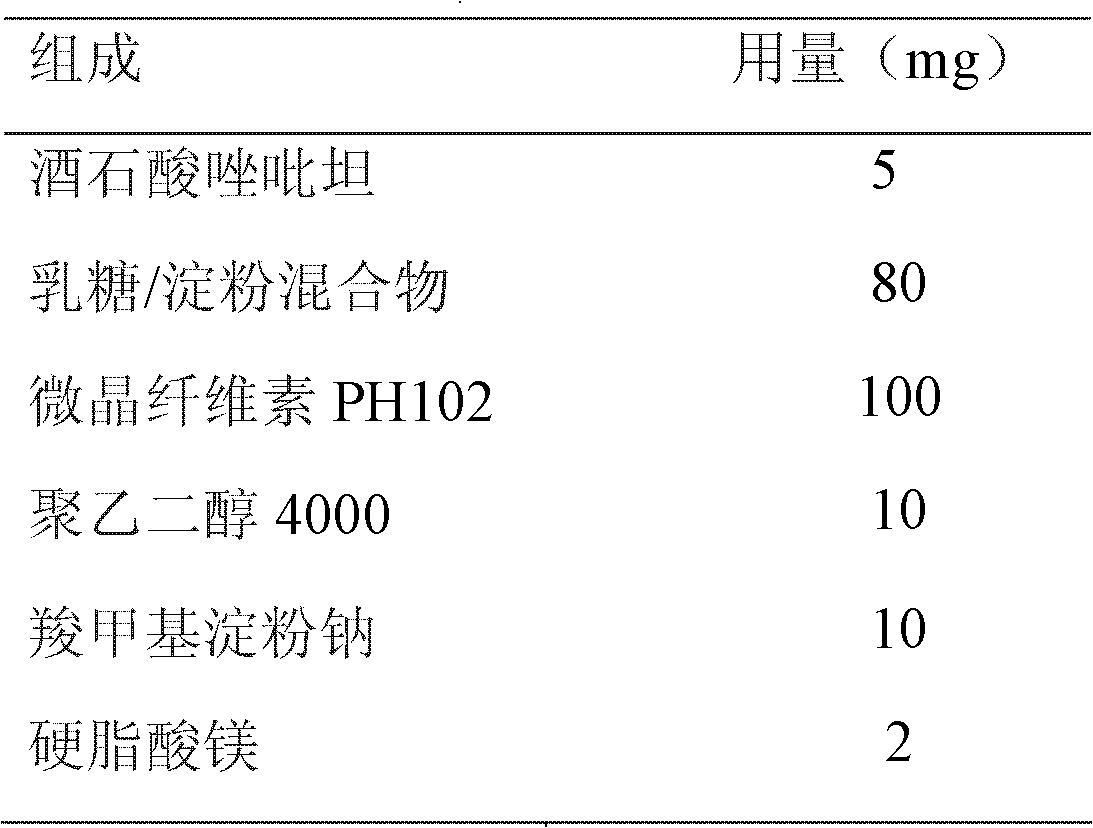
[0022] Ordinary drug-containing immediate-release powder:
[0023] prescription:
[0024]
[0025] Process: mix the prescribed amount of zolpidem tartrate, lactose / starch mixture, microcrystalline cellulose PH102, polyethylene glycol 4000, and sodium carboxymethyl starch, add the prescribed amount of magnesium stearate, mix evenly, and obtain .
[0026] Enteric-coated immediate-release pellets
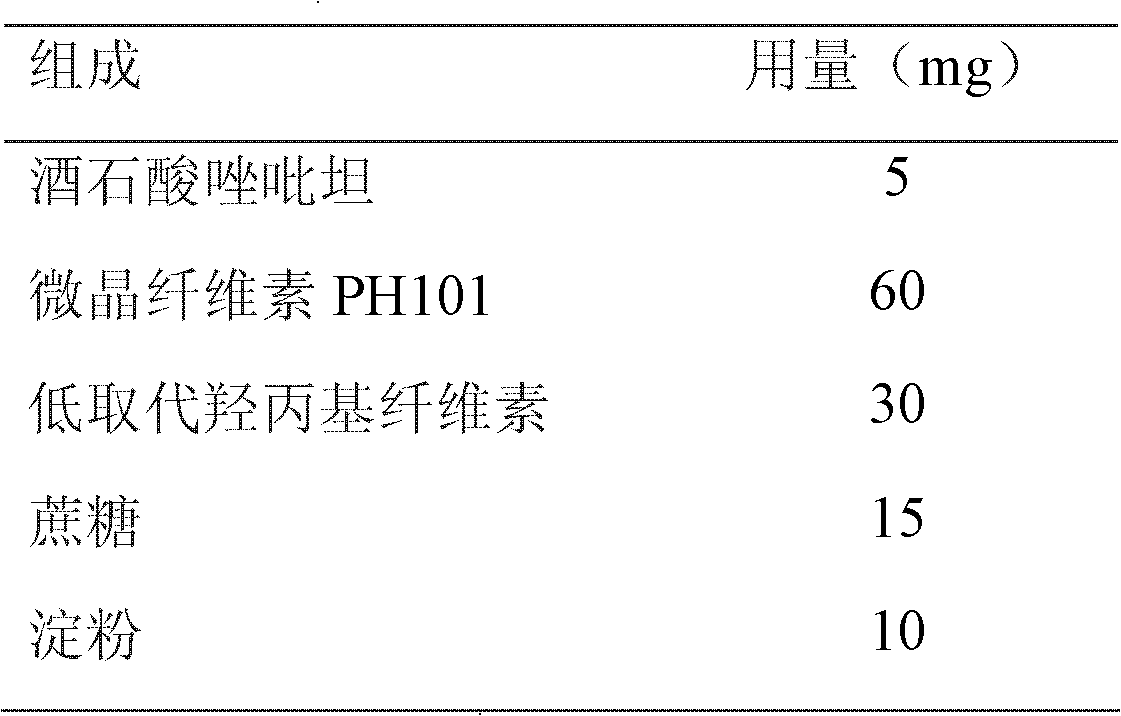
[0027] Ball core
[0028]
[0029] Enteric coating
[0030]
[0031] Process: mix the prescribed amount of zolpidem tartrate, microcrystalline cellulose PH101, low-substituted hydroxypropyl cellulose, and sucrose evenly, add 10% starch slurry to make a soft material, extrude and spheronize, and make an immediate-release pellet core, sausage Dissolving coating, coating weight gain 10-15%.
[0032] Mix the prepared quick-release granules and enteric-coated granules evenly, and press into tablet...
Embodiment 2
[0037] Example 2: Biphasic release tablet containing 12.5 mg zolpidem tartrate
[0038] Ordinary medicated immediate release powder
[0039]
[0040] Process: mix the prescribed amount of zolpidem tartrate, mannitol, microcrystalline cellulose PH200, and croscarmellose sodium, add the prescribed amount of calcium stearate, mix evenly, and obtain the product.
[0041] Enteric-coated immediate-release pellets
[0042] Ball core
[0043]
[0044] Enteric coating
[0045]
[0046] Process: Mix the prescription amount of zolpidem tartrate, microcrystalline cellulose PH101, low-substituted hydroxypropyl cellulose, and lactose evenly, add 0.1% carboxymethyl cellulose sodium soft material, extrude (35rpm) and spheronize (600rpm ), made into quick-release ball cores, enteric-coated, and the weight of the coating increased by 25-30%.
[0047] Mix the prepared quick-release granules and enteric-coated granules evenly, and press into tablets to obtain.
[0048]Adopt Chinese ...
Embodiment 3
[0052] Example 3: Biphasic Release Tablet Containing 12.5 mg Zolpidem Tartrate
[0053] Ordinary drug-containing immediate-release powder:
[0054]
[0055] Process: mix the prescription amount of zolpidem tartrate, lactose, microcrystalline cellulose PH102, and sodium carboxymethyl starch evenly, add the prescription amount of magnesium stearate, mix evenly, and get ready.
[0056] Enteric-coated immediate-release pellets:
[0057]
[0058] Enteric coating:
[0059]
[0060] Process: mix the prescription amount of zolpidem tartrate, microcrystalline cellulose PH101, crospovidone, and ethyl methyl cellulose evenly, add 10% pregelatinized starch slurry to make a soft material, extrude and spheronize, and make Immediate release pellet core, enteric coating, coating weight gain 15-25%.
[0061] Mix the prepared quick-release granules and enteric-coated granules evenly, and press into tablets to obtain.
[0062] Adopt Chinese Pharmacopoeia (2010 Edition) Appendix II I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com