Nifedipine sustained release tablets and preparation method thereof
A technology of nifedipine and nifedipine, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as difficult bioequivalence, unresearched release behavior, etc. The effect of increasing the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 20mg nifedipine sustained-release tablet prescription (300,000 / batch) and preparation method thereof:
[0070]
[0071] Preparation Process:
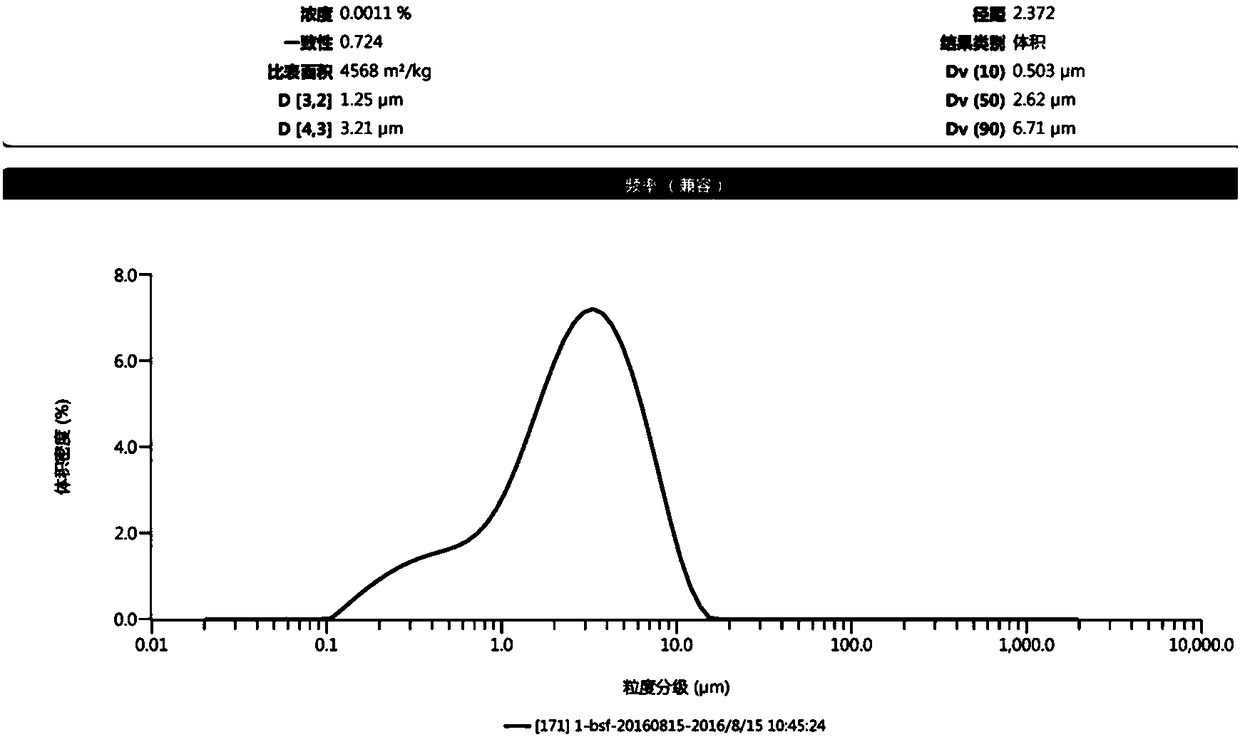
[0072] Pass the prescribed amount of microcrystalline cellulose, hypromellose, and hypromellose through an 80-mesh sieve, and control the particle size of ethyl cellulose to D 90 200 ~ 300um, standby; hypromellose, hypromellose, ethyl cellulose mixed uniformly as blocker composition; blocker composition, anhydrous calcium hydrogen phosphate and raw materials (D 90 5 ~ 10um) into the wet granulator, after stirring at speed I for 10 minutes, add microcrystalline cellulose and continue stirring for 5 minutes; add an appropriate amount of 95% ethanol to dissolve Tween 80, stir at speed I and chop at speed II during the liquid addition process 4min, out of the pan; after drying in an oven at 30°C for 1h, increase the temperature to 50°C and continue drying for 1h until the water content is lower than 3%, and granulate; add dry gr...
Embodiment 2
[0074] 20mg nifedipine sustained-release tablet prescription (300,000 / batch) and preparation method thereof:
[0075]
[0076] Preparation Process:
[0077] Pass the prescribed amount of microcrystalline cellulose, hypromellose, and hypromellose through an 80-mesh sieve, and control the particle size of ethyl cellulose to D 90 200 ~ 300um, standby; hypromellose, hypromellose, ethyl cellulose mixed uniformly as blocker composition; blocker composition, anhydrous calcium hydrogen phosphate and raw materials (D 90 5 ~ 10um) into the wet granulator, after stirring at speed I for 10 minutes, add microcrystalline cellulose and continue stirring for 5 minutes; add an appropriate amount of 95% ethanol to dissolve Tween 80, stir at speed I and chop at speed II during the liquid addition process 4min, out of the pan; after drying in an oven at 30°C for 1h, increase the temperature to 50°C and continue drying for 1h until the water content is lower than 3%, and granulate; add dry gr...
Embodiment 3
[0079] 20mg nifedipine sustained-release tablet prescription (300,000 / batch) and preparation method thereof:
[0080]
[0081] Preparation Process:
[0082] Pass the prescribed amount of microcrystalline cellulose, hypromellose, and hypromellose through an 80-mesh sieve, and control the particle size of ethyl cellulose to D 90200 ~ 300um, standby; hypromellose, hypromellose, ethyl cellulose mixed uniformly as blocker composition; blocker composition, anhydrous calcium hydrogen phosphate and raw materials (D 90 5 ~ 10um) into the wet granulator, after stirring at speed I for 10 minutes, add microcrystalline cellulose and continue stirring for 5 minutes; add an appropriate amount of 95% ethanol to dissolve Tween 80, stir at speed I and chop at speed II during the liquid addition process 4min, out of the pan; after drying in an oven at 30°C for 1h, increase the temperature to 50°C and continue drying for 1h until the water content is lower than 3%, and granulate; add dry gra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com