Double-layer sustained-release nifedipine tablet and preparation method thereof
A bilayer, nifedipine technology, applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, pill delivery, etc., can solve the problems of short half-life, large fluctuation of blood drug concentration, peak-to-valley phenomenon, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] prescription:
[0048] Immediate release layer:
3.0g
[0049] Crospovidone XL
4.5g
Microcrystalline Cellulose 101
16.5g
Lactose Granulac200
30.0g
Povidone K30
4.5g
Micropowder silica gel
1.0g
0.3g
water
Appropriate amount
Slow release layer:
Nifedipine
7.0g
Hypromellose 100LV
18.0g
Microcrystalline Cellulose 101
49.0g
Povidone K30
7.0g
Micropowder silica gel
2.0g
0.7g
water
Appropriate amount
production
1000 pieces
[0050] Preparation:
[0051] (1) Material preparation: Nifedipine (Tianjin Zhongan Pharmaceutical Co., Ltd.) was micronized to a particle size range of 10-20 μm, and each component was weighed according to the prescription amount.
[0052] (2) Preparation of immediate-release granu...
Embodiment 2-8
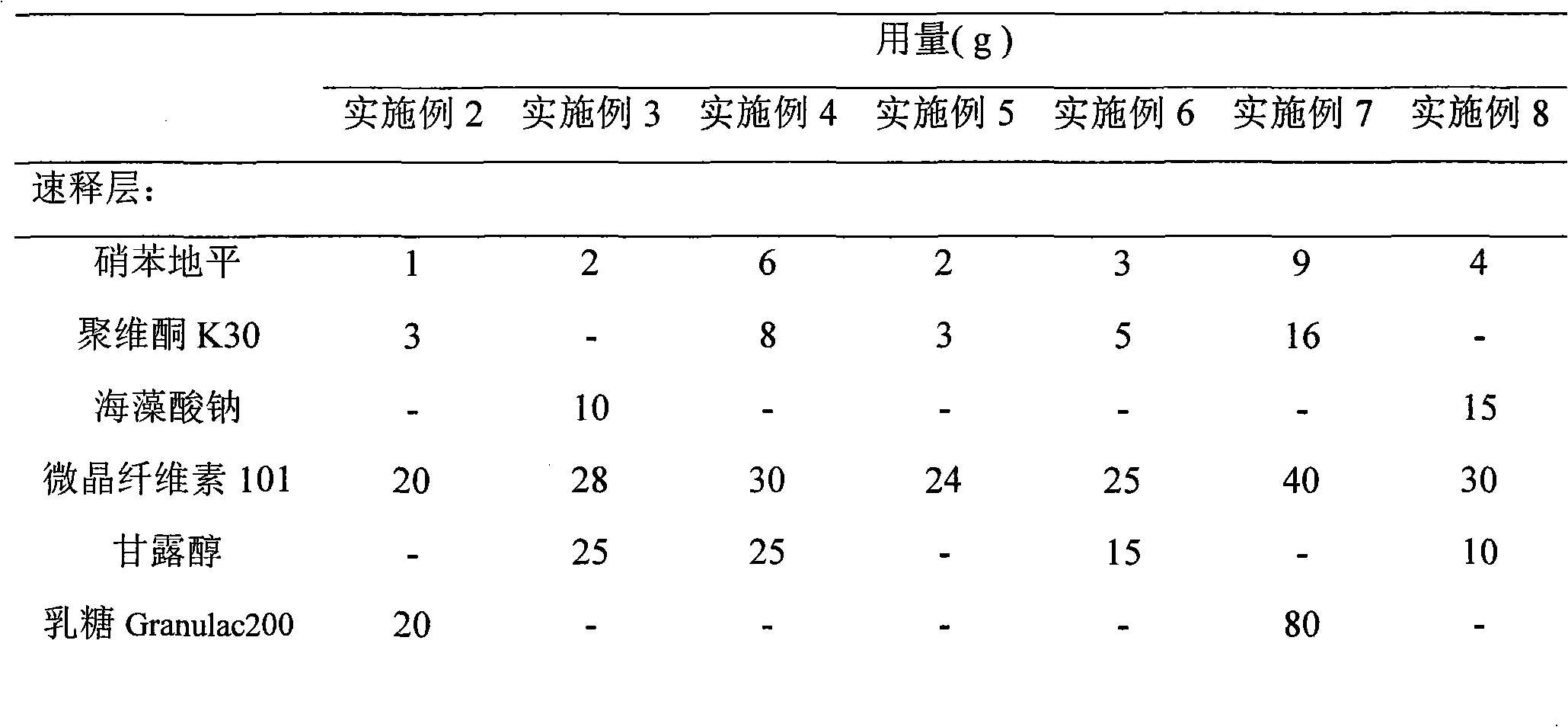
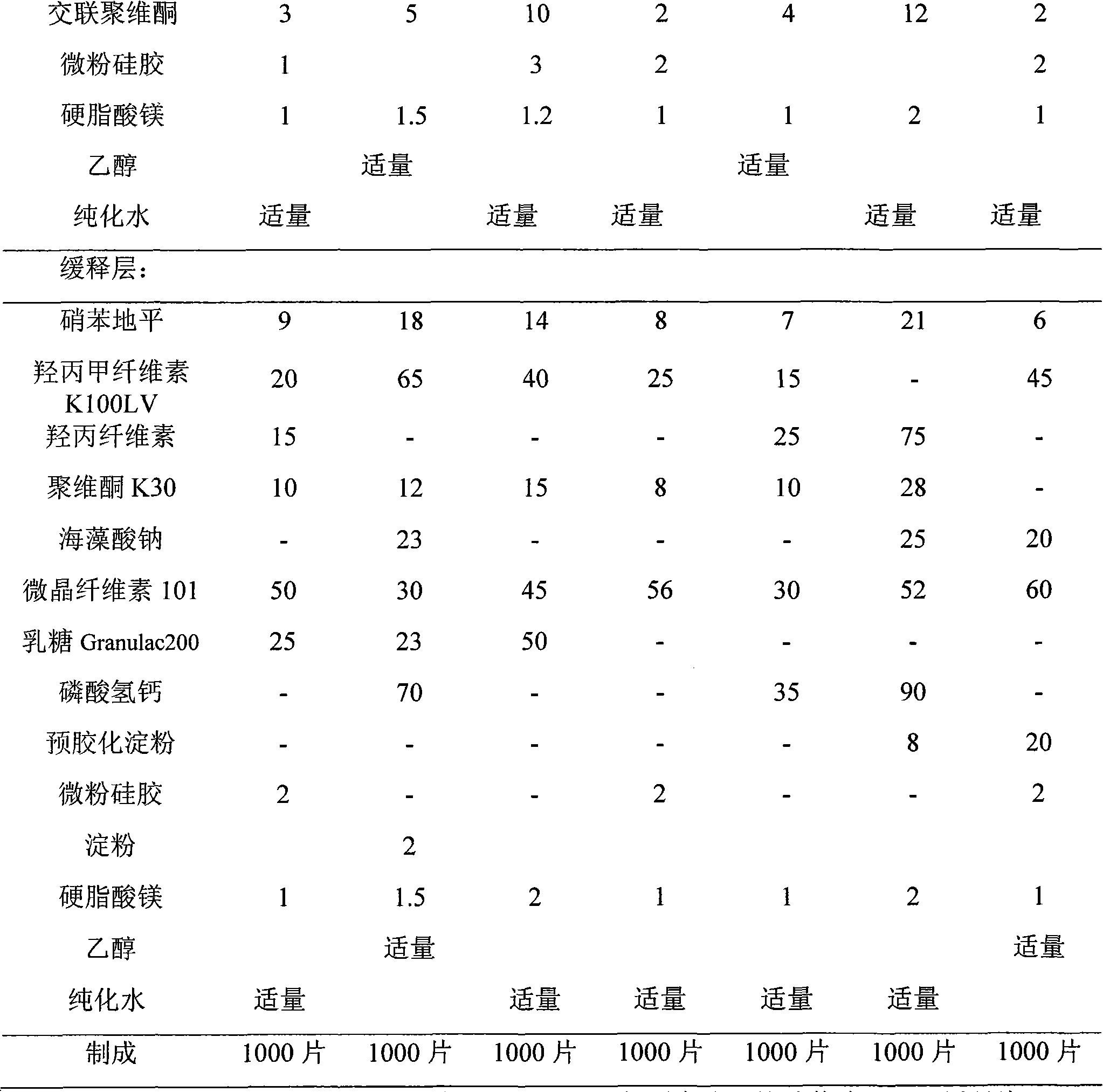
[0070] prescription:
[0071]
[0072]
[0073] Preparation method: with embodiment 1, obtain the nifedipine double-layer sustained-release tablet of the present invention in the same manner as embodiment 1. Among them, mannitol was used from French Roquette Company, starch was used from Shenyang Yian Pharmaceutical Excipients Co., Ltd., sodium alginate was used from Yantai Xingfu Seaweed Industry Co., Ltd., and hydroxypropyl cellulose was used from Japan Soda Co., Ltd. Products, calcium hydrogen phosphate use the product of JRS in Germany, and pregelatinized starch use the product of Shanghai Colorcon Coating Technology Co., Ltd.
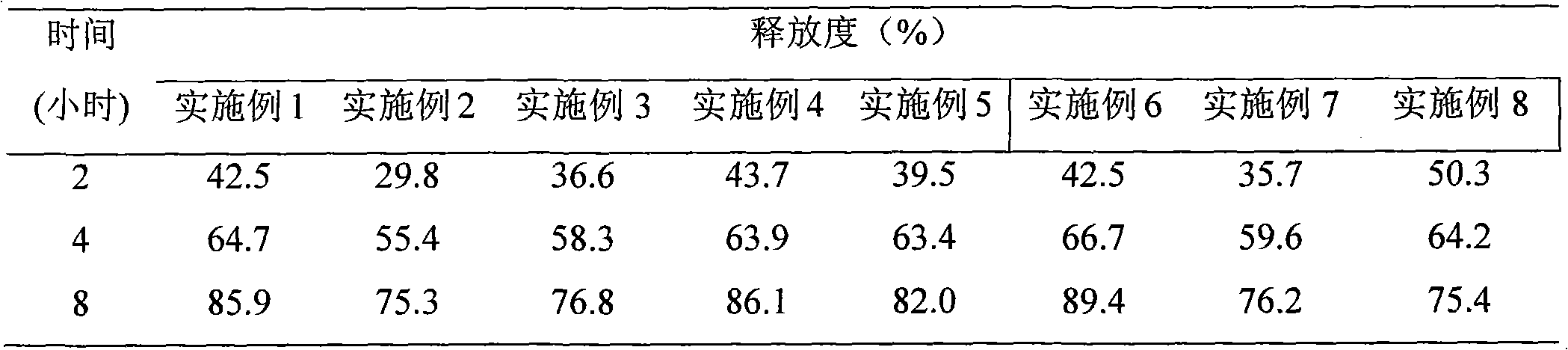
[0074] In order to investigate the in vitro release effect of the present invention, according to the release assay ("Chinese Pharmacopoeia" edition in 2005 two appendix X D first method), adopt the second method device of dissolution assay (operation in dark place), with hydrochloric acid solution (9 → 1000) 1000ml is the release medium, th...
Embodiment 9
[0079] Three batches of samples (batch numbers 090810, 090811, and 090812, each batch of 10,000 pieces) were amplified by the prescription process of Example 1, and the data of the three batches of amplified experiments are summarized in Table 2. in:
[0080] The release measurement method is the same as above.
[0081] Content determination method Avoid light operation. Take 20 tablets of this product, accurately weigh, grind finely, accurately weigh an appropriate amount (approximately equivalent to 30mg of nifedipine), put it in a mortar, add 2ml of chloroform, grind it, and quantitatively transfer it to a 100ml measuring bottle with absolute ethanol. , dilute to the mark with absolute ethanol, shake well, filter, discard the initial filtrate, accurately measure 5ml of the subsequent filtrate, put it in a 50ml measuring bottle, add absolute ethanol and release to the mark, shake well, and measure the UV-visible spectrophotometry Method (two appendix IV A of Chinese Pharma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com