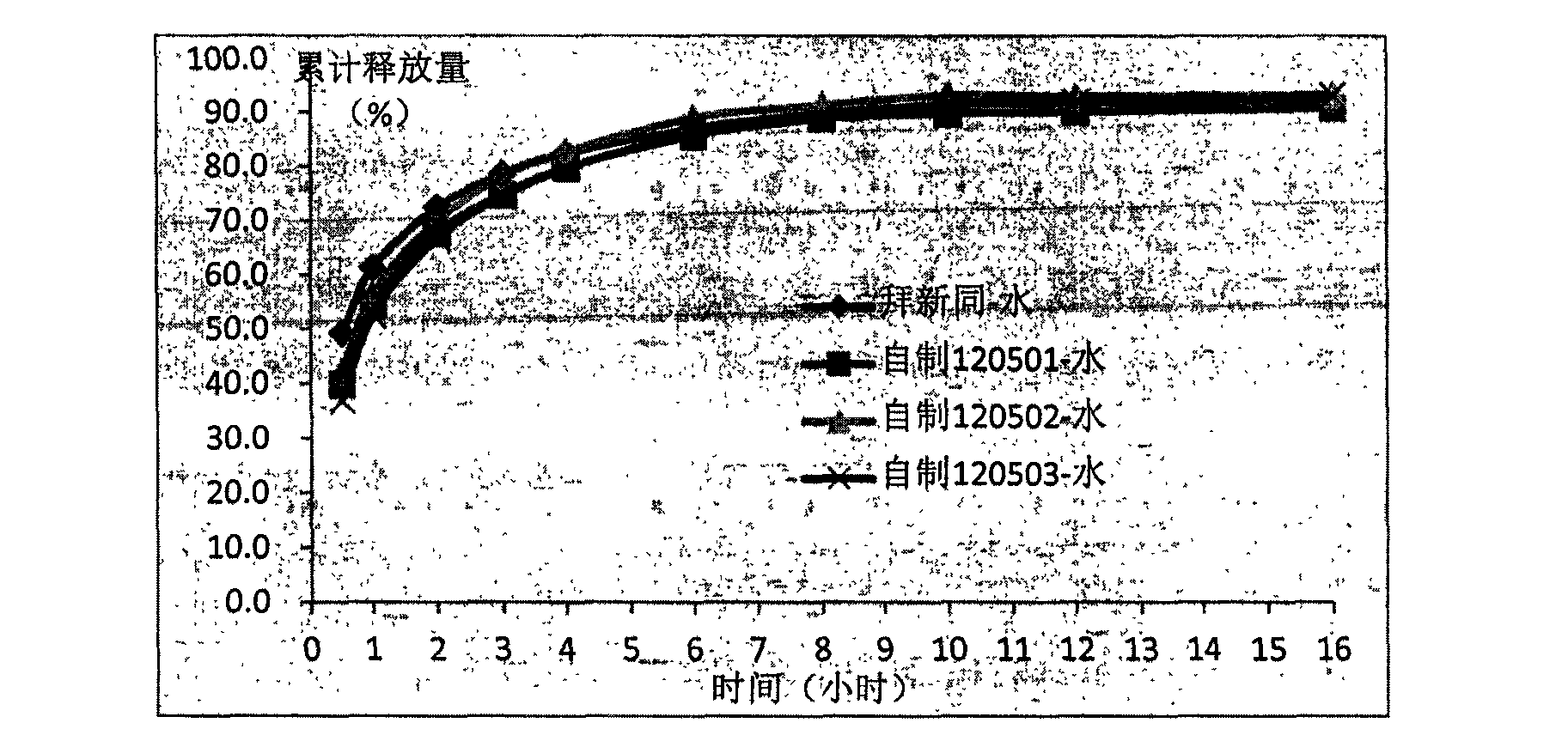
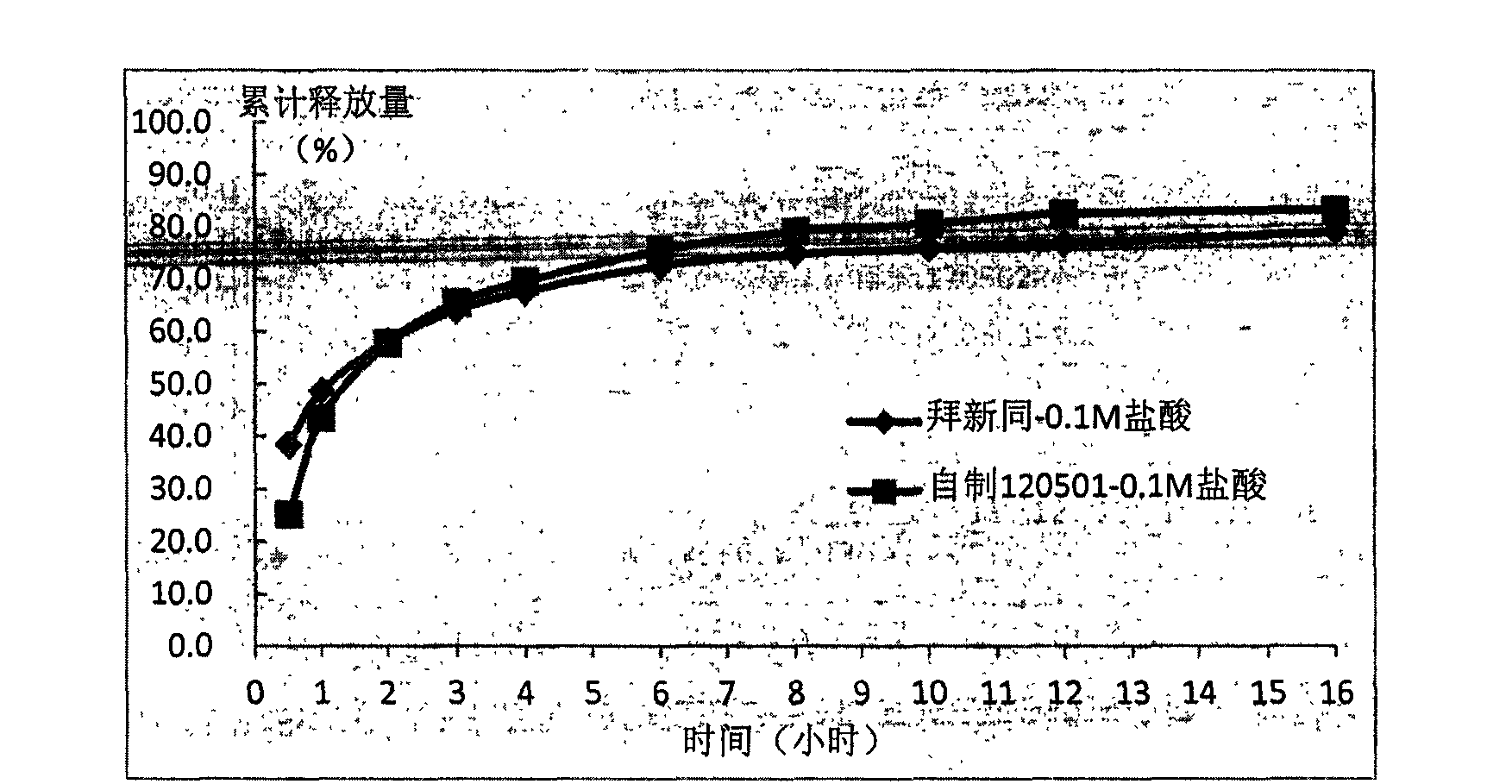
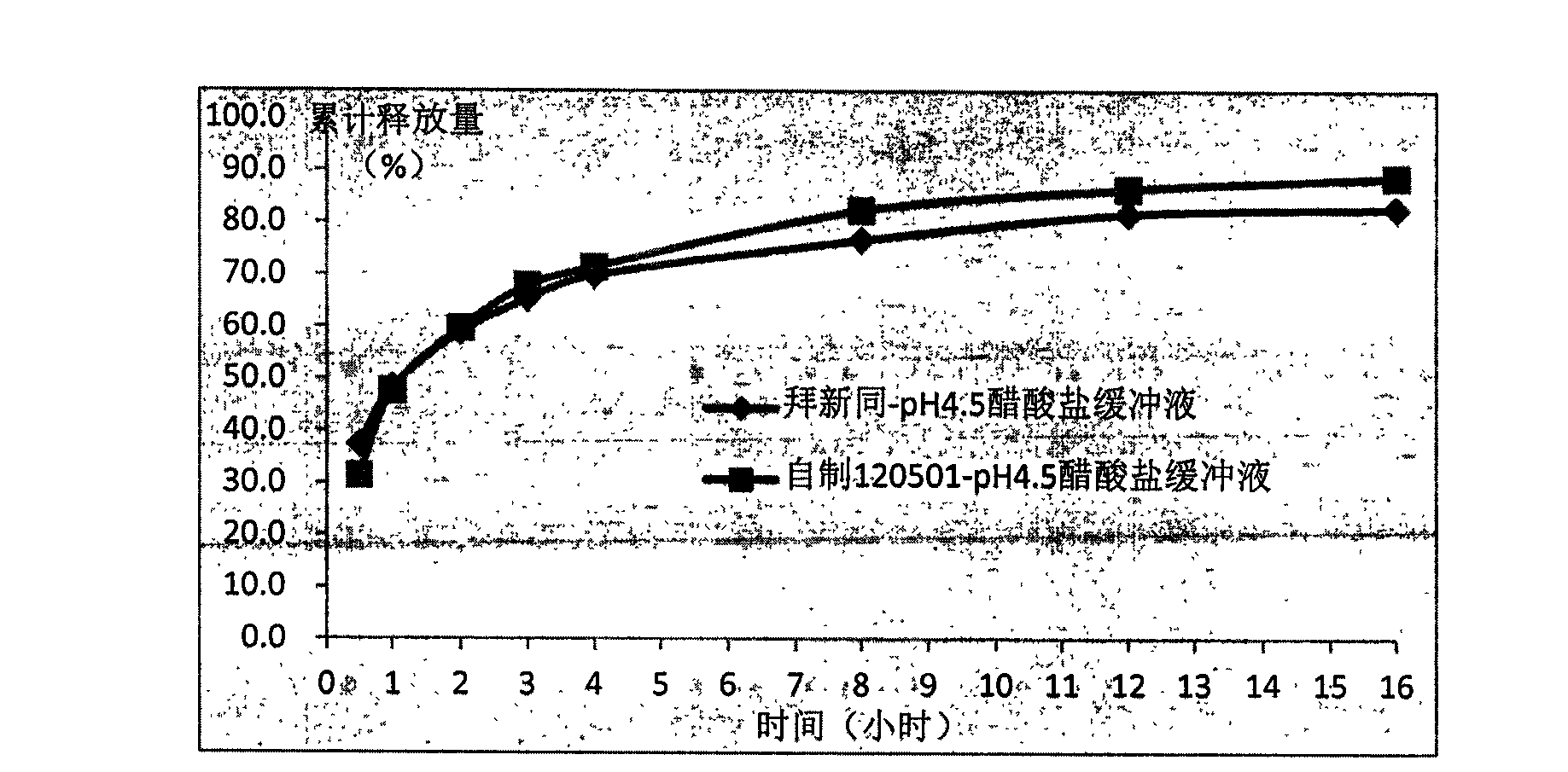
Sustained release tablet for treating cardiovascular diseases and preparation method thereof
A sustained-release tablet and cardiovascular technology, which is applied in the field of sustained-release tablets for the treatment of cardiovascular diseases and its preparation, can solve the problems of not containing immediate-release ingredients, consuming large energy, and harming patients, so as to avoid production equipment and production processes , Conducive to environmental protection, to avoid the effect of potential harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Embodiment 1: Nifedipine sustained release tablet
[0123] Immediate Release Granules:
[0124]
[0125] Slow-release granules:
[0126]
[0127] Additional accessories:
[0128] Magnesium stearate 0.6g
[0129] Microcrystalline Cellulose PH102 12g
[0130] Weigh nifedipine, lactose, microcrystalline cellulose PH101 and starch according to the prescription, place them in the granulator, turn on the main paddle at 200r / m, and the cutter at 1500r / m, stir for 4 minutes, and mix well; weigh the starch for slurry , add purified water, stir evenly, add polysorbate 80, stir evenly, heat and stir, and prepare 13% starch slurry as an adhesive; add the adhesive to the uniformly mixed material, turn on the main paddle 200r / m. Cutter 1500r / m, stir for 30 seconds, stop the machine to clean the pot wall, continue stirring for 1 minute, and discharge; the soft material is granulated through a 20-mesh sieve, dried at 60°C, granulated with a 20-mesh sieve, and the instant-rel...
Embodiment 2
[0131] Embodiment 2: Nifedipine sustained release tablet
[0132] Immediate Release Granules:
[0133]
[0134]
[0135] Slow-release granules:
[0136]
[0137] Additional accessories:
[0138] Magnesium Stearate 1.0g
[0139] Microcrystalline Cellulose PH102 9.6g
[0140] Weigh nifedipine, lactose, microcrystalline cellulose PH101 and starch according to the prescription, place them in the granulator, turn on the main paddle at 100r / m, and the cutter at 2500r / m, stir for 8 minutes, and mix well; weigh the starch for pulp , add purified water, stir evenly, add polysorbate 80, stir evenly, heat and stir, and prepare 6% starch slurry as an adhesive; add adhesive to the uniformly mixed material, turn on the main paddle 100r / m. Cutter 3000r / m, stir for 80 seconds, stop the machine to clean the pot wall, continue to stir for 0.6 minutes, and discharge; the soft material is granulated through a 12-mesh sieve, dried at 70°C, and granulated with a 30-mesh sieve to obtai...
Embodiment 3
[0141] Embodiment 3: Nifedipine sustained release tablet
[0142] Immediate Release Granules:
[0143]
[0144] Slow-release granules:
[0145]
[0146] Additional accessories:
[0147] Magnesium stearate 0.4g
[0148] Microcrystalline Cellulose PH102 14.4g
[0149] Weigh nifedipine, lactose, microcrystalline cellulose PH101 and starch according to the prescription, place them in the granulator, turn on the main paddle at 250r / m, and the cutter at 600r / m, stir for 3 minutes, and mix well; weigh the starch for pulp , add purified water, stir evenly, add polysorbate 80, stir evenly, heat and stir, and prepare a 15% starch slurry as an adhesive; add the adhesive to the uniformly mixed material, turn on the main paddle 250r / m. Cutter 600r / m, stir for 20 seconds, stop the machine to clean the pot wall, continue stirring for 4 minutes, and discharge; the soft material is granulated through a 30-mesh sieve, dried at 50°C, granulated with a 12-mesh sieve, and the instant-re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com