Nifedipine double-layer osmotic pump tablet and preparation method thereof
A double-layer, osmotic pump tablet technology, applied in coatings, pharmaceutical formulations, medical preparations with non-active ingredients, etc., to achieve the effect of improving the release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Drug-containing layer (per tablet):
[0051]
[0052] (2) Booster layer prescription (per tablet):
[0053]
[0054] (3) Composition of semi-permeable membrane coating solution (per 1000 tablets):
[0055]
[0056] (4) Composition of moisture-proof coating solution (per 1000 tablets):
[0057] OPADRY 03B640002 PINK Appropriate amount
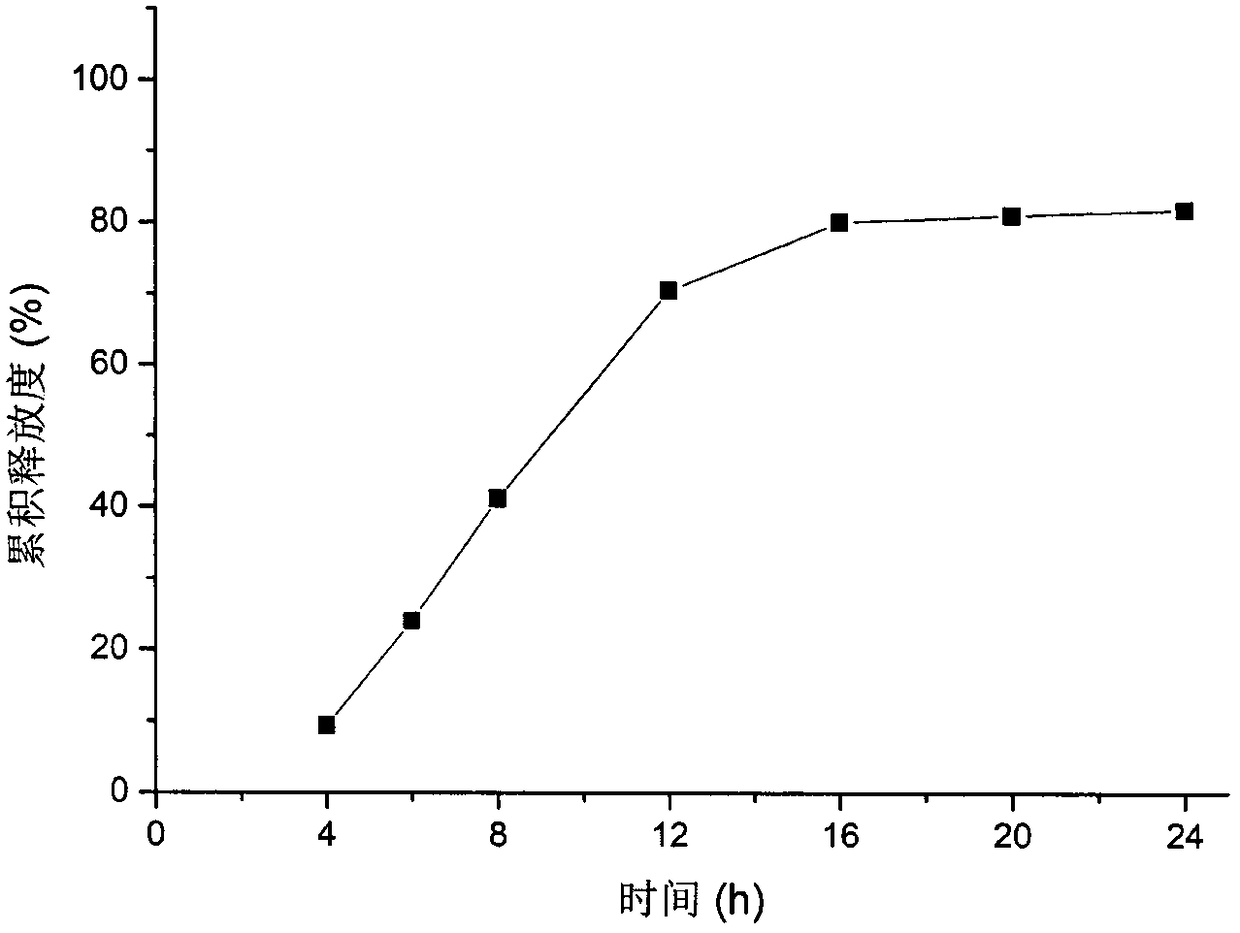
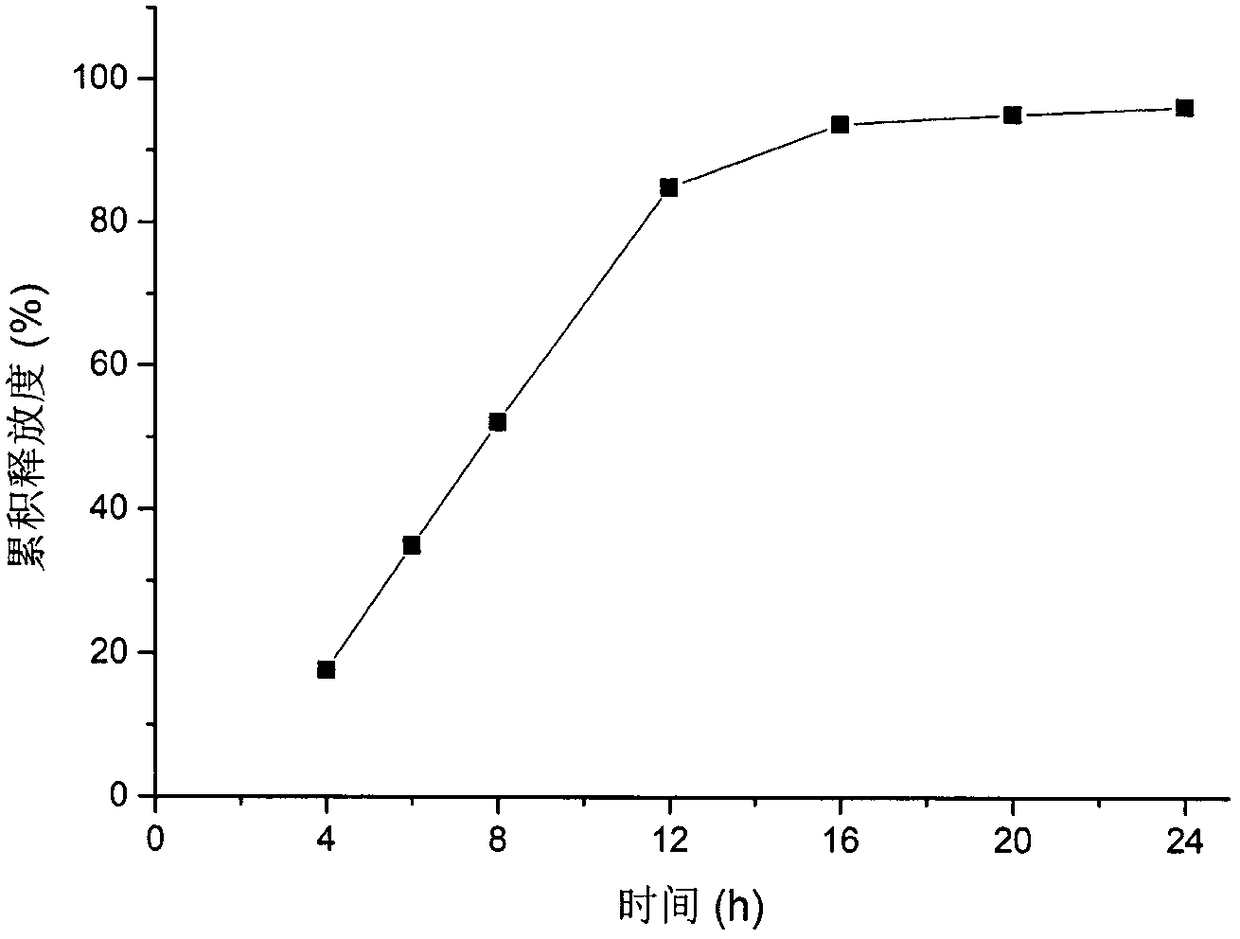
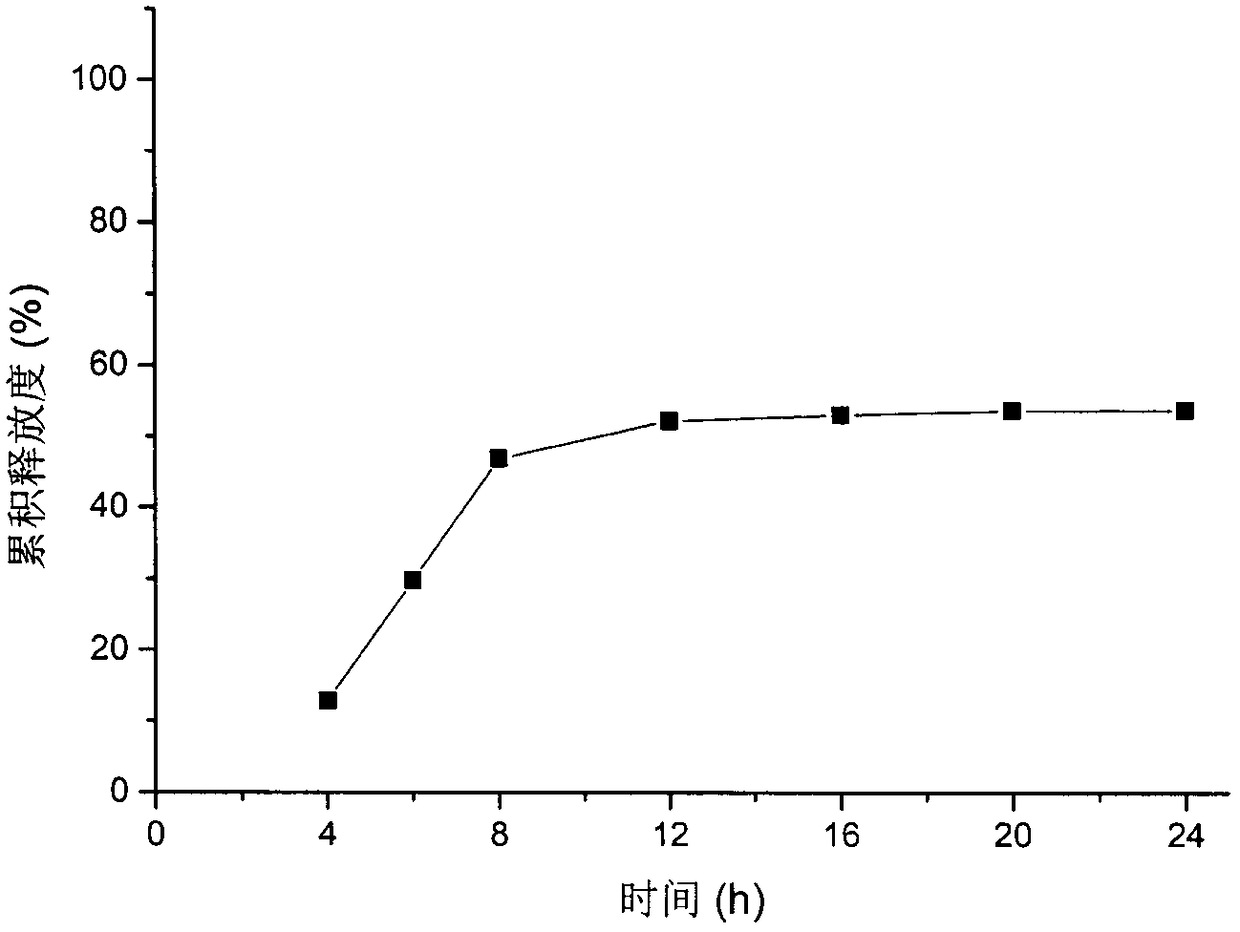
[0058] For the release curve of the formulation in CN 100563638C see figure 1 , the nifedipine double-layer osmotic pump tablet release rate of embodiment 1 sees figure 2 , it can be seen that adding an appropriate amount of sodium lauryl sulfate in the drug-containing layer can significantly improve the release end point of nifedipine, so that the release of nifedipine is complete.
Embodiment 1- Embodiment 9
[0059] Embodiment 1-Example 9 Preparation process of nifedipine double-layer osmotic pump tablet:
[0060] 1. Preparation of drug-containing layer mixture: avoid light. The raw and auxiliary materials are passed through a 60-mesh sieve, and the components of the drug-containing layer in the prescribed amount are mixed uniformly in the principle of equal increase without adding a lubricant, and then mixed with the prescribed amount of magnesium stearate for 5 minutes, and set aside.
[0061] 2. Preparation of the mixture of the booster layer: mix the components of the booster layer in the prescribed amount without adding a lubricant, and mix them uniformly according to the principle of equal increase, and then mix them with the prescribed amount of magnesium stearate for 5 minutes, and set aside.
[0062] 3. Tablet compression: keep away from light. The mixture of the drug-containing layer and the mixture of the booster layer is compressed into a double-layer tablet core with a ...
Embodiment 2
[0068] (1) Drug-containing layer (per tablet):
[0069]
[0070] (2) Booster layer prescription (per tablet):
[0071]
[0072] (3) Composition of semi-permeable membrane coating solution (per 1000 tablets):
[0073]
[0074] (4) Composition of moisture-proof coating solution (per 1000 tablets):
[0075] OPADRY 03B640002 PINK Appropriate amount
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Film diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com