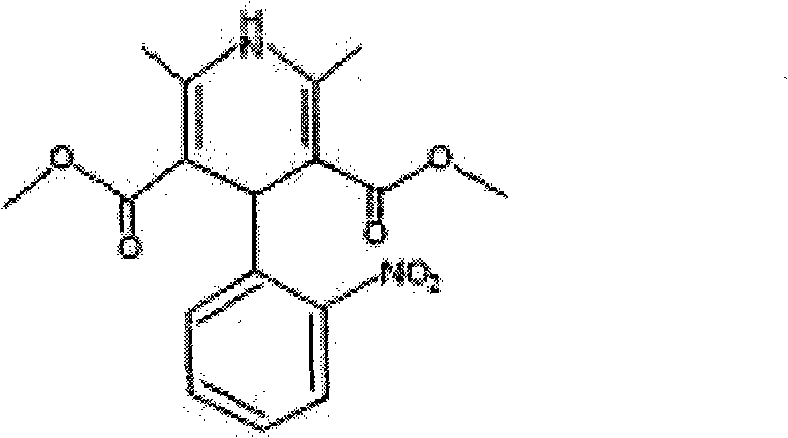
Nifedipine sustained-release tablet
A nifedipine, gentle technology, applied in cardiovascular system diseases, pharmaceutical formulations, drug combinations, etc., can solve problems such as unavoidable side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Nifedipine 15g
[0024] Lactose 30g
[0025] PVP k29 / 32 10g
[0026] Low-substituted hypromellose 30g
[0027] Sodium Alginate 60g
[0029] Preparation: Grind the remaining excipients of nifedipine separately, pass through 80 mesh sieve; mix nifedipine and sodium alginate evenly; granulate with PVP K29 / 30 ethanol solution, dry, and granulate with 14 mesh sieve; The rest of the excipients are mixed evenly; compressed into tablets.
Embodiment 2
[0031] Nifedipine 30g
[0032] Lactose 40g
[0033] PVP k29 / 32 10g
[0034] Crosslinked PVP 34g
[0035] Hydroxypropyl Methyl Cellulose 50g
[0037] Preparation: Grind the remaining excipients of nifedipine separately, pass through 80 mesh sieve; mix nifedipine and hydroxypropyl methylcellulose evenly; granulate with PVP K29 / 30 ethanol solution, dry, and pass through 14 mesh sieve Whole grain; mixed with other excipients; compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com