Nifedipine composition and preparation method thereof
A technology of nifedipine and a composition, applied in the field of medicine, can solve the problems of low reproducibility, too slow release, residual organic solvent, etc., and achieve the effects of good ductility and thermoplasticity, stable release curve, and continuous release curve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
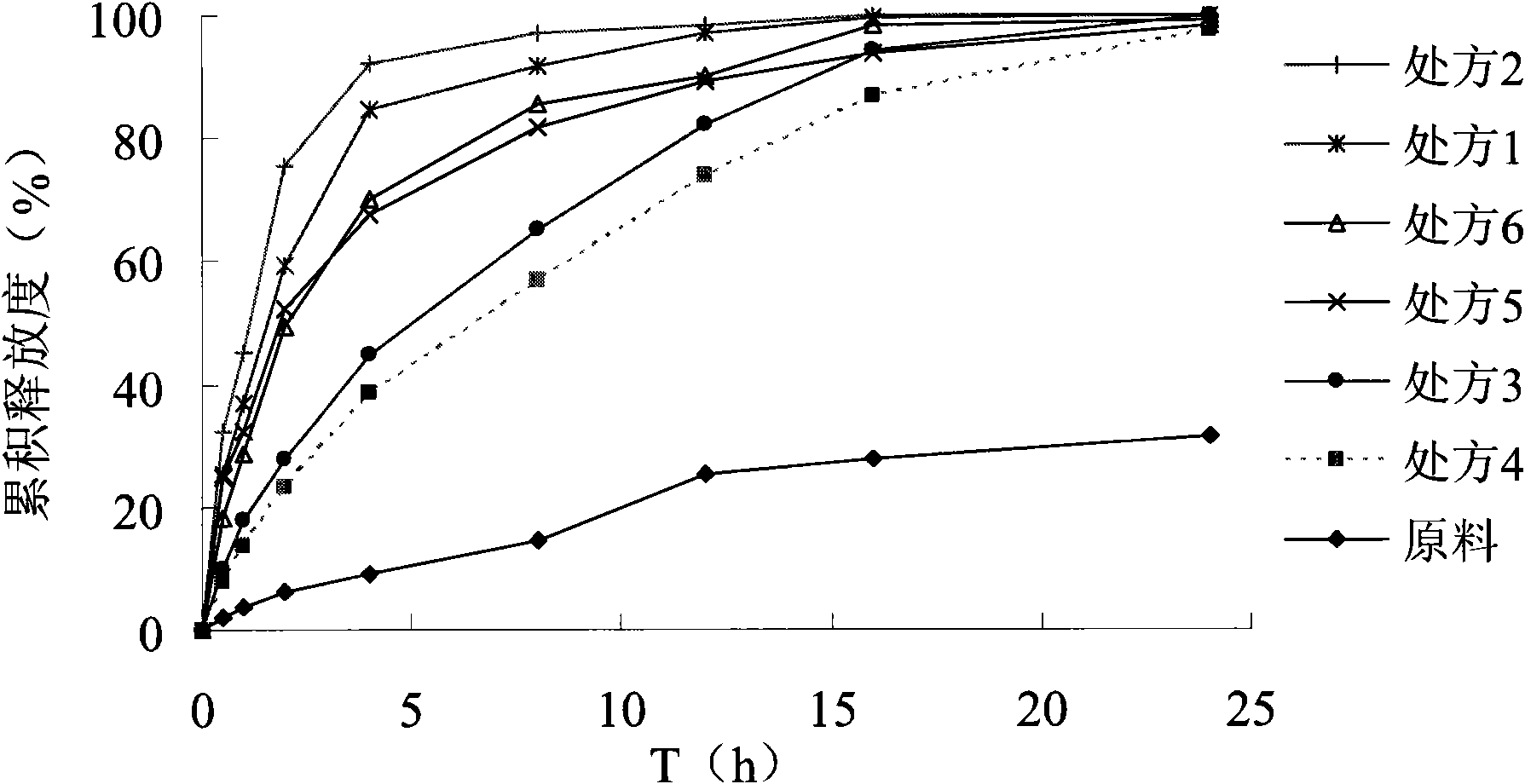
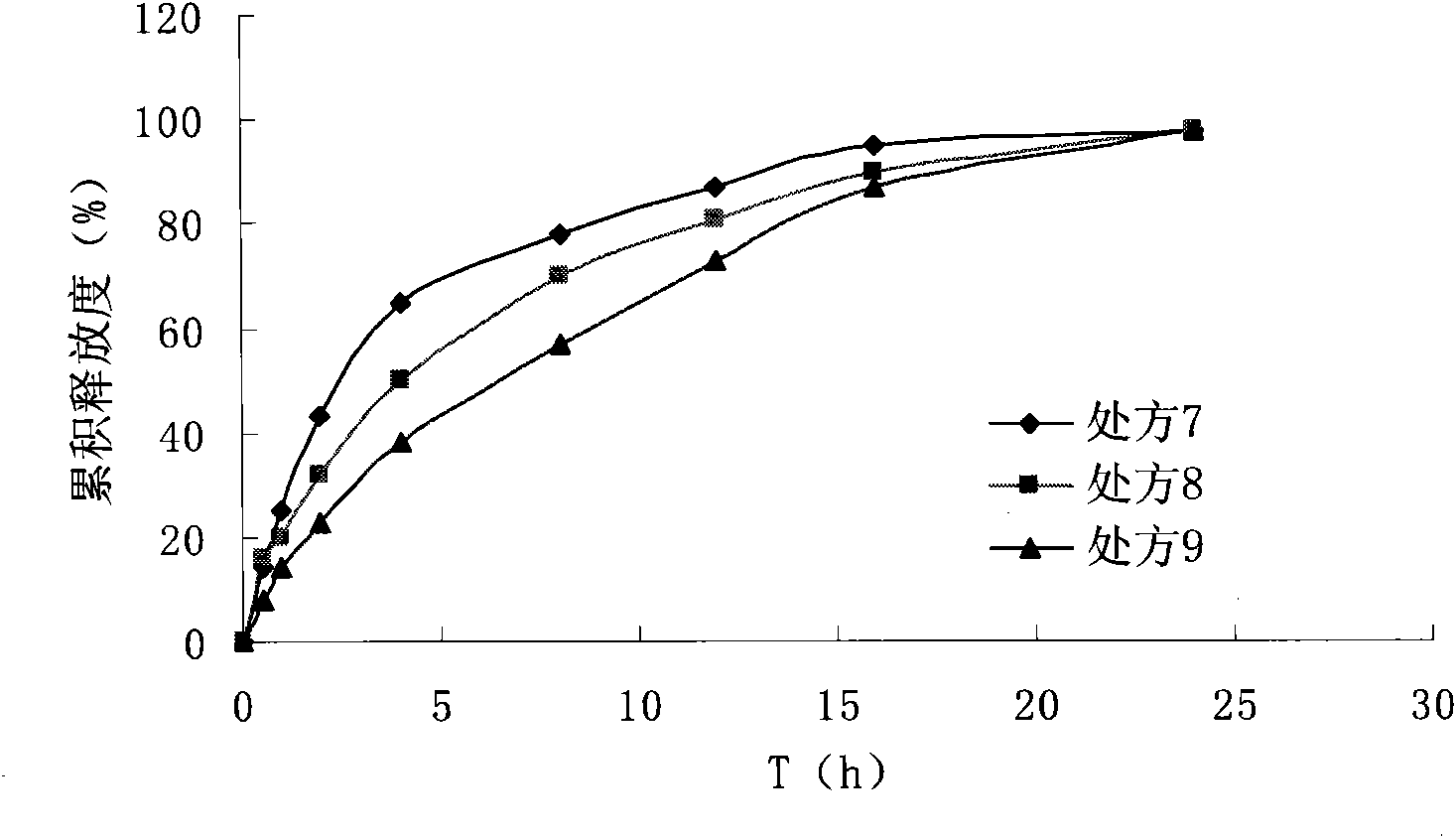
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 crude drug tablet
[0023] Prescription: Nifedipine 10g, microcrystalline cellulose 180g, magnesium stearate 1.5g. Preparation method: mix the raw and auxiliary materials evenly, and directly compress into tablets.
Embodiment 2
[0024] Embodiment 2 solvent method prepares solid dispersion
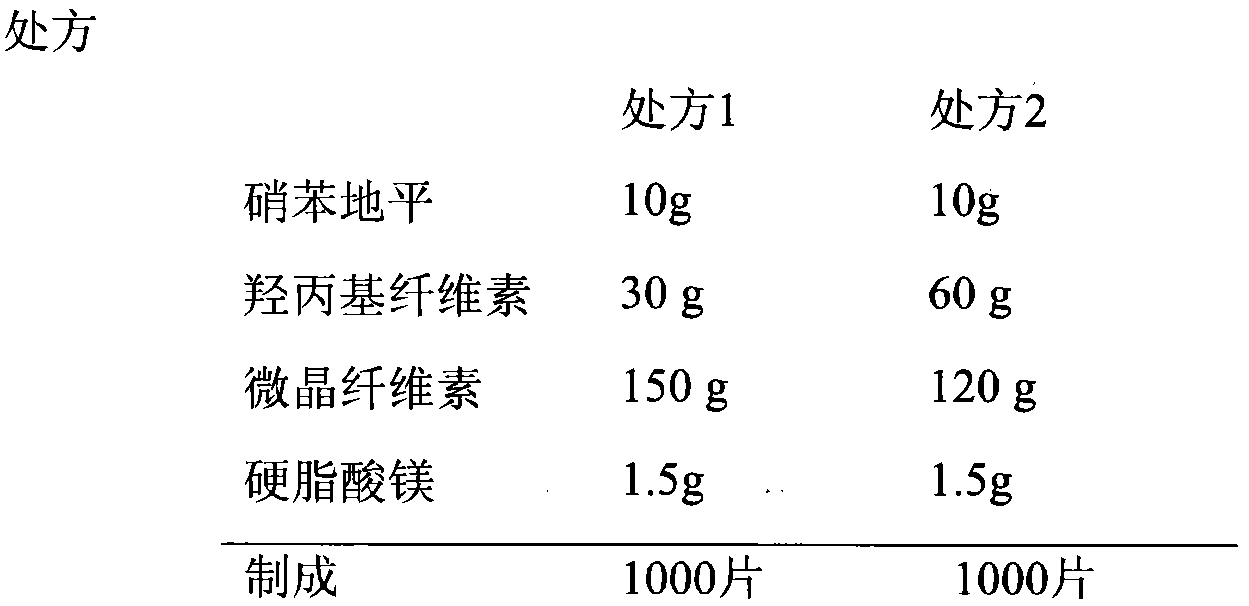
[0025]
[0026] Preparation
[0027] The prescribed amount of nifedipine, hydroxypropyl cellulose ( HPC EF, Ashland, USA) and microcrystalline cellulose excipients were dissolved in absolute ethanol, mixed evenly, removed from absolute ethanol, dried at 60°C for 24 hours, pulverized to a suitable particle size, mixed with magnesium stearate, and compressed into tablets. Get nifedipine.
Embodiment 3
[0028] Embodiment 3 hot-melt extrusion method prepares solid dispersion
[0029]
[0030] Extrusion temperature setting: 100°C-120°C-120°C-120°C-120°C
[0031] Preparation
[0032] The prescribed amount of nifedipine, hydroxypropyl cellulose ( HPC EF, Ashland, USA) and microcrystalline cellulose auxiliary materials are placed in the co-rotating twin-screw extruder TE-20 (Nanjing Coperon Keya Co., Ltd.), and the temperature from each section of the machine to the head is set respectively, and the screw speed is 40RPM. The extruded product is cooled, crushed to a suitable particle size, mixed with magnesium stearate, and pressed into tablets to obtain nifedipine tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com