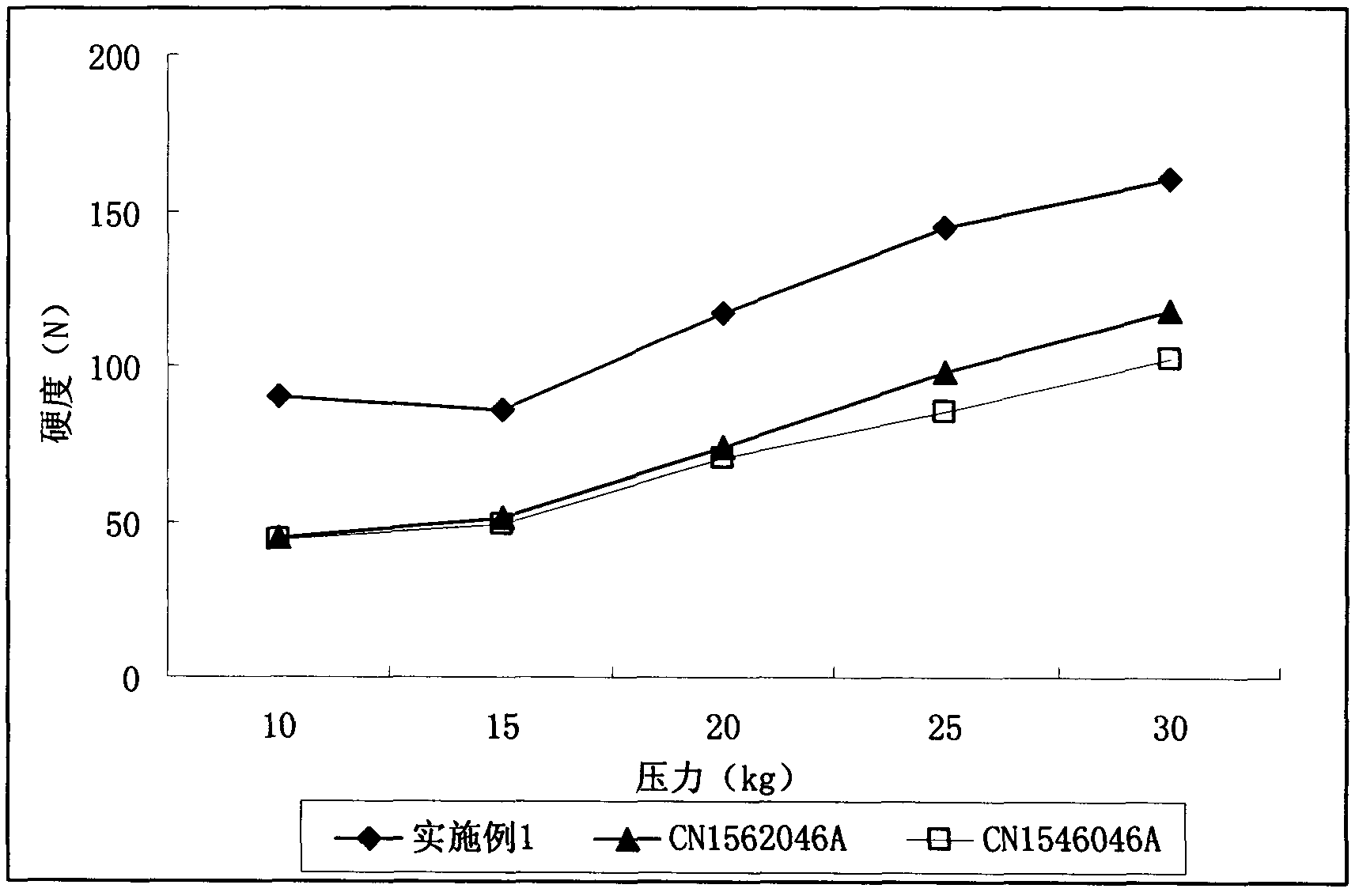
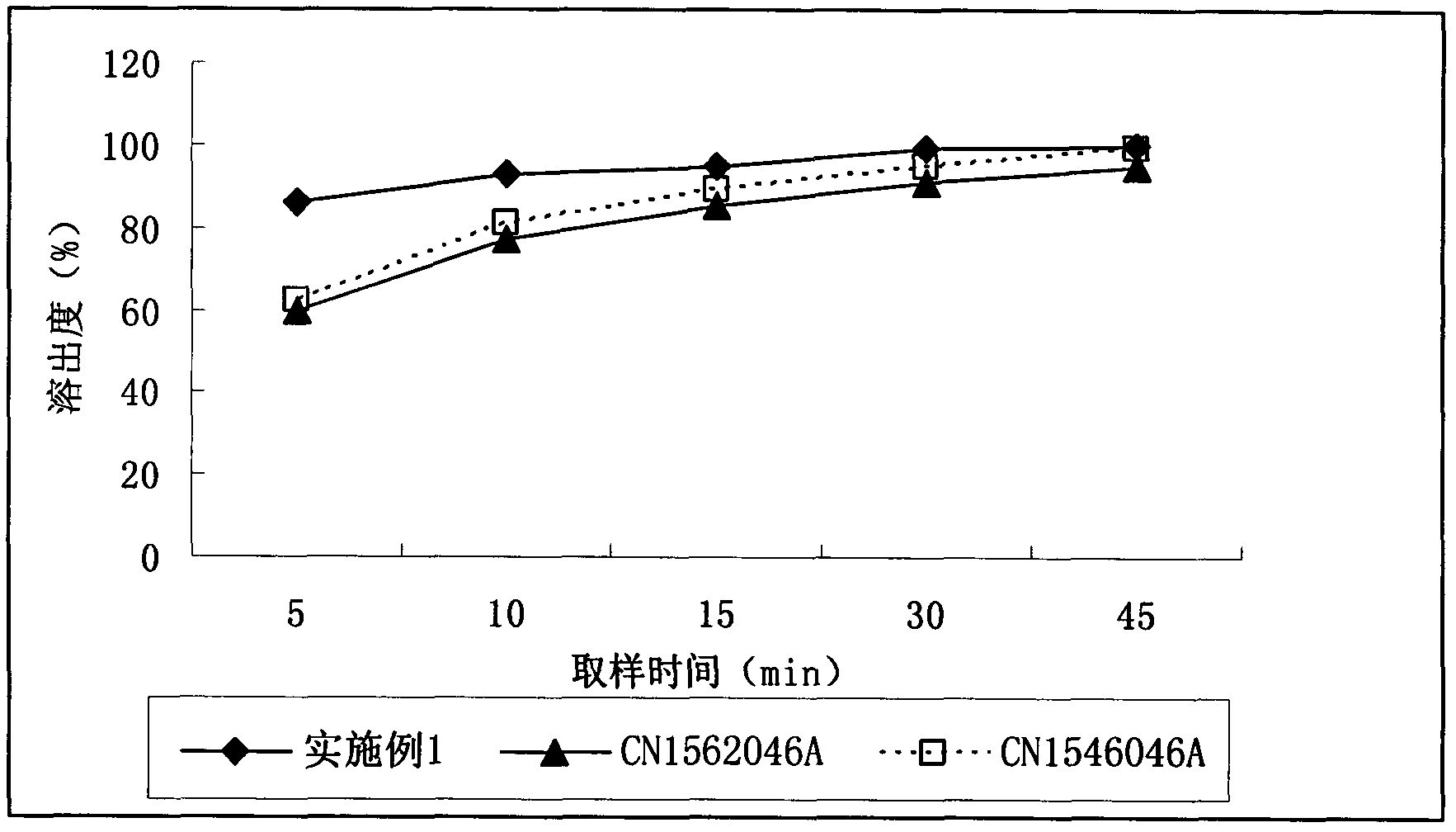
Adefovir dipivoxil pharmaceutical composition
A technology for adefovir dipivoxil and pharmaceutical preparations, applied in the field of medicine, can solve the problems of the influence of preparation stability, adefovir dipivoxil intolerance to humidity and heat, and the dissolution rate needs to be improved, and achieves excellent dispersion uniformity and excellent release properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
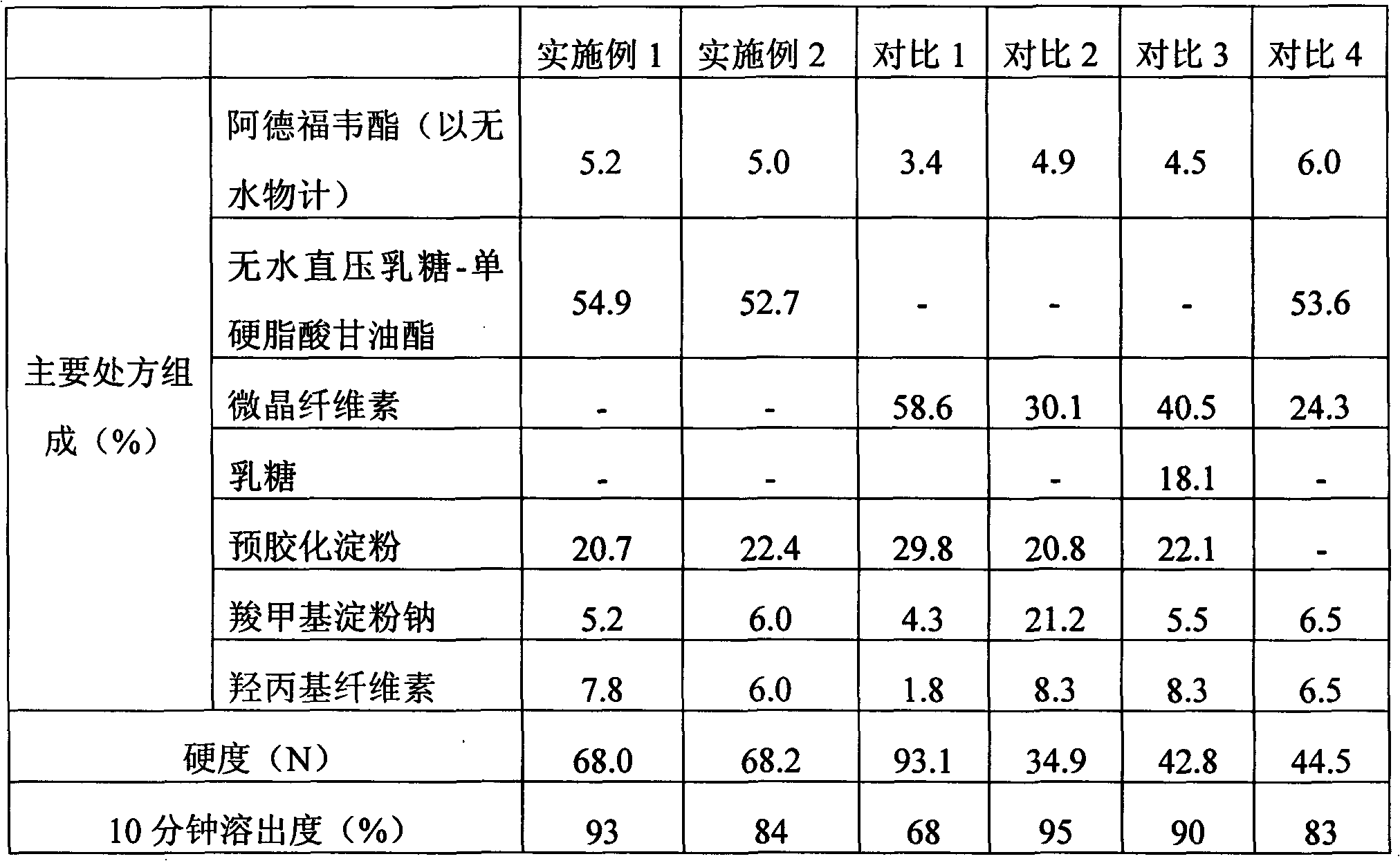
Embodiment 1
[0040]
[0041] Preparation method: Mix adefovir dipivoxil, anhydrous direct-pressed lactose-glyceryl monostearate, pregelatinized starch, hydroxypropyl cellulose and sodium carboxymethyl starch, and press the tablet.
Embodiment 2
[0043]
[0044] Preparation method: Mix adefovir dipivoxil, anhydrous direct-pressed lactose-glyceryl monostearate, pregelatinized starch, hydroxypropyl cellulose and sodium carboxymethyl starch, and press the tablet.
Embodiment 3
[0046]
[0047] Preparation method: Mix adefovir dipivoxil, anhydrous direct-pressed lactose-glyceryl monostearate, pregelatinized starch, hydroxypropyl cellulose and sodium carboxymethyl starch, and press the tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com