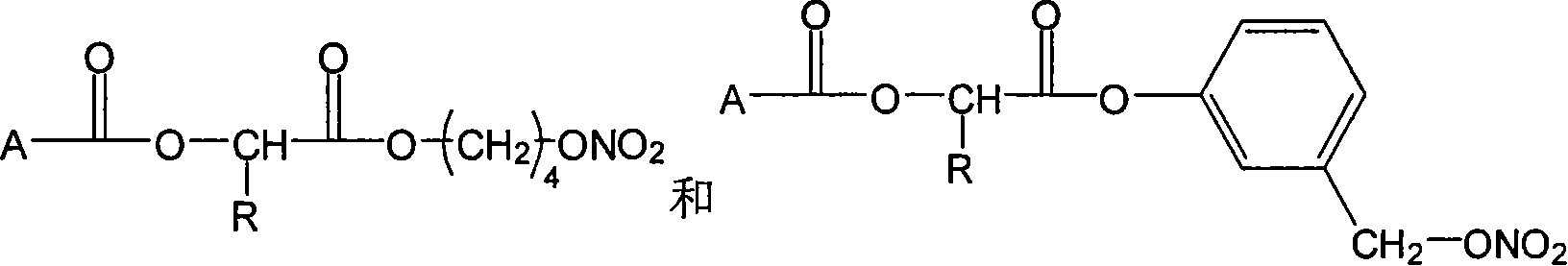
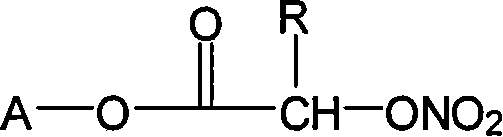
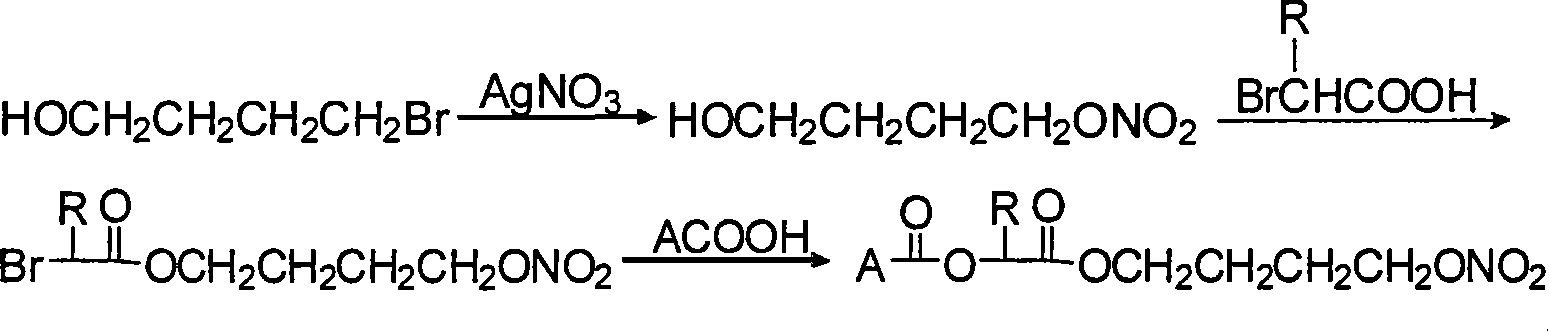Nonsteroidal antiinflammatories with nitric oxide donors and its preparation method
A non-steroidal anti-inflammatory drug, nitric oxide technology, applied in anti-inflammatory agents, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve problems such as bleeding or even perforation, large side effects of the digestive tract, thromboembolism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Ricofilone m-nitroxymethylphenyl acetate
[0033]
[0034] Dissolve 9.5 g (0.05 mol) of m-hydroxybenzyl bromide in 100 ml of acetonitrile, add 10 g (0.06 mol) of silver nitrate, and stir in the dark at 25°C for 18 hours, filter off the solid, spin the filtrate to dryness, and purify it by column chromatography, and use petroleum ether -eluted with ethyl acetate to obtain m-nitrooxymethylphenol, 4.5 g of yellow oily matter, yield 53%. Dissolve the oil in 30ml of dichloromethane, add 3.7g (0.027mol) of bromoacetic acid and 6g of DCC, stir at 25°C for 8 hours, filter, make sand from the filtrate, and purify by column to obtain m-nitroxymethylbenzene bromoacetic acid Ester, light brown oil 4.7g.
[0035] Dissolve 0.1g of sodium metal (0.00435mol) in 10ml of methanol, add 1.6g (0.0042mol) of ricofilon, spin dry after dissolving, dissolve the solid in 10ml of DMF, drop into the bromoacetic acid m-nitroxymethylphenyl 1.2g (0.0042mol) and DMF10ml solution, st...
Embodiment 2
[0036] Embodiment 2: Diclofenac m-nitrooxymethylphenyl acetate
[0037]
[0038] Dissolve 0.2g (0.0087mol) of sodium metal in 20ml of methanol, add 2.5g (0.00845mol) of diclofenac, spin dry after dissolving, dissolve the solid in 20ml of DMF, drop into 2.5g of m-nitrooxymethylphenyl bromoacetate ( 0.00845mol) and 20ml of DMF, stirred at 25°C for 4 hours, added 120ml of ethyl acetate, washed three times with water, dried, sanded, and eluted with petroleum ether-ethyl acetate to obtain 1.4g of a yellowish solid. (MS-ESI(+): 505[M+H] + ;IR: 1770, 1732, 1633, 1277cm -1 ; NMR (CDCl 3 ): 8.23 (s, 1H), 6.82-7.40 (m, 11H), 6.00 (s, 2H), 4.82 (s, 2H), 3.76 (s, 2H)).
Embodiment 3
[0039] Embodiment 3: m-nitroxymethylphenyl acetate of clomethoxib
[0040]
[0041] Dissolve 0.25g of sodium metal (0.0109mol) in 10ml of methanol, add 3g of clomethoxib (0.0102mol), spin dry after dissolution, dissolve the solid in 10ml of DMF, and drop into 3g of m-nitrooxymethylphenyl bromoacetate (0.0102mol) and DMF10ml solution, stirred at 20°C for 4 hours, added 40ml of ethyl acetate, washed three times with water, made sand after drying, and eluted with petroleum ether-ethyl acetate to obtain 0.6g of yellow oil. (MS-ESI(+): 503[M+H] + ;IR: 1740, 1635, 1270cm -1 ; NMR (CDCl 3 ): 9.03(s, 1H), 6.52-7.38(m, 10H), 6.20(s, 2H), 4.98(s, 2H), 3.86(s, 2H)), 2.25(s, 3H)).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com