Synthetic method of saccharide-containing copolymer with biological specific recognition performance
A synthesis method and copolymer technology, which is applied in the measurement of color/spectral characteristics, etc., can solve the problems of difficult synthesis of dendritic sugar-containing polymers, and achieve the effects of reliable polymer synthesis methods, broadening the scope of practical applications, and clear structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
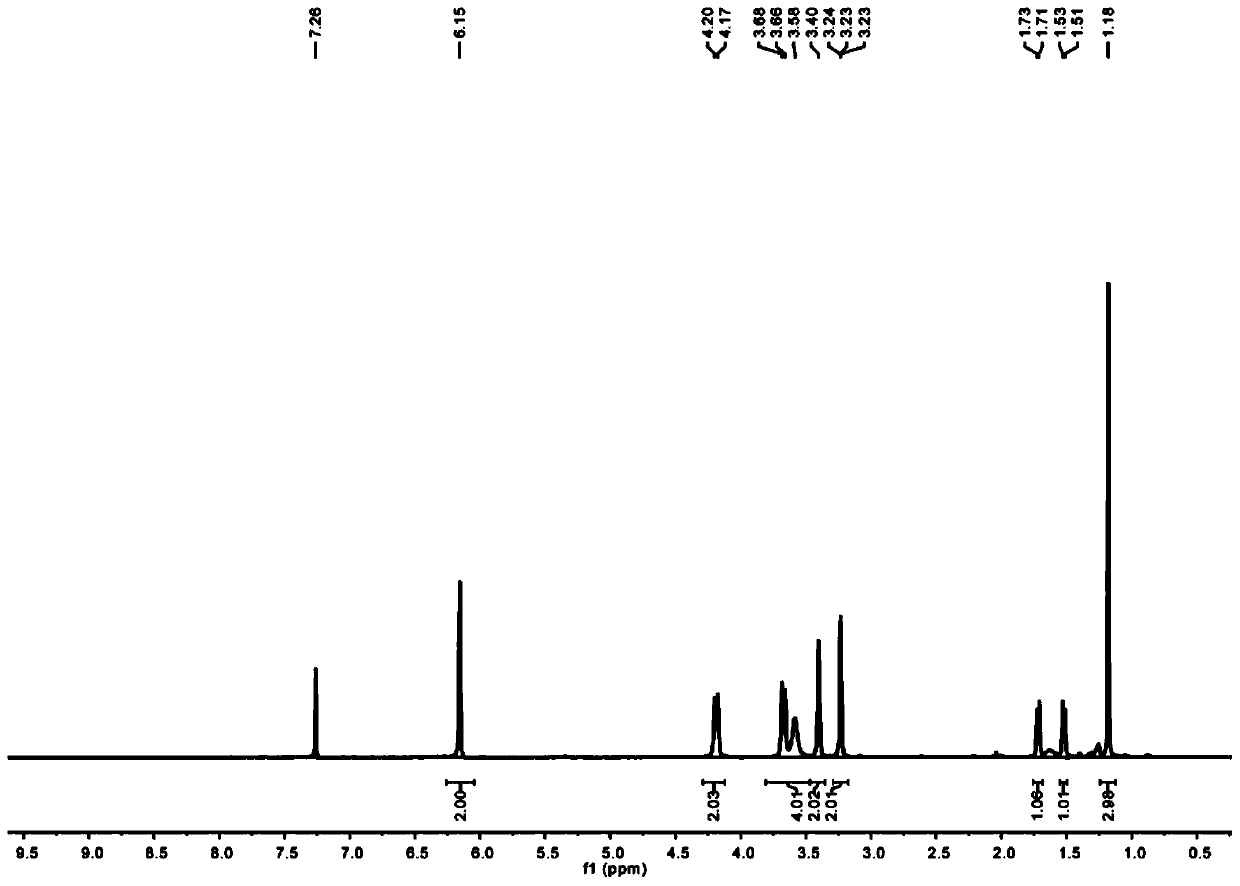
[0054] In this example, alkyne-terminated norbornene derivatives and azide-containing sugar compounds were used as raw materials to synthesize and prepare by copper-catalyzed azide-terminal alkyne [3+2] cycloaddition (CuAAC) reaction. NB-2Man-OAc and NB-2Gal-OAc two types of sugar-containing polymer monomers with protective groups, followed by ring-opening metathesis polymerization (ROMP) to prepare block copolymers P((( NB-2Man-OH)-b-(NB-2Gal-OH)).
[0055] 1. Synthesis of norbornene dicarboxamide propylene glycol NB-2OH (2)
[0056] Take the three-neck round bottom bottle cooled after high temperature treatment, add cis-5-norbornene-exo-2,3-dicarboxylic acid anhydride (1g, 6.1mmol) and aminomethylpropanediol (0.96g, 9.15mmol), and add 30mL of toluene. Connect the h-type water separator at the mouth of the bottle, connect the serpentine condenser above it, and reflux the reaction in an oil bath at 135°C for 16 hours. After the reaction, the toluene is distilled off under re...
Embodiment 2
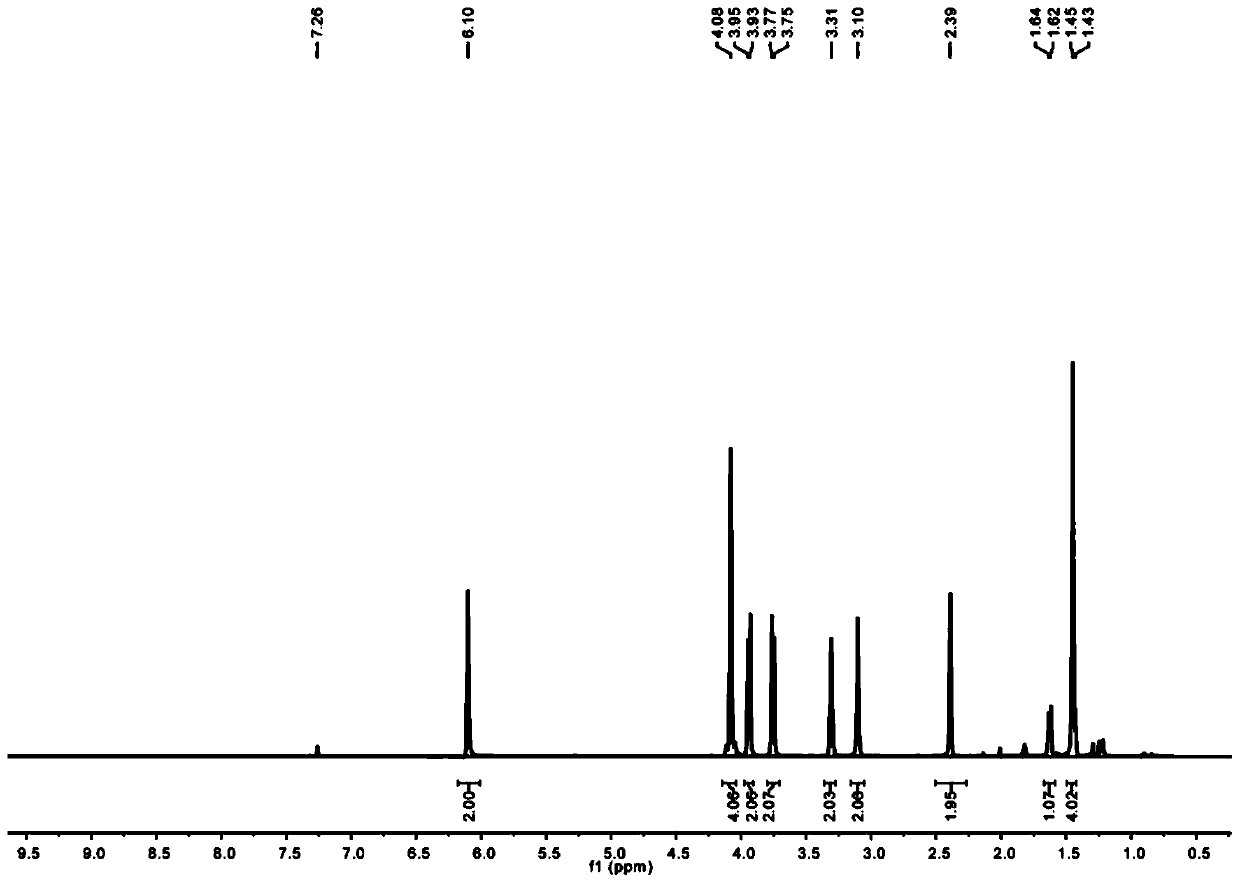
[0072] In this example, alkyne-terminated norbornene derivatives and azide-containing sugar compounds were used as raw materials to synthesize and prepare by copper-catalyzed azide-terminal alkyne [3+2] cycloaddition (CuAAC) reaction. NB-2Man-OAc and NB-2Gal-OAc two types of sugar-containing polymer monomers with protective groups, followed by ring-opening metathesis polymerization (ROMP) to prepare block copolymers P((( NB-2Man-OH)-b-(NB-2Gal-OH)).
[0073] 1. Synthesis of norbornene dicarboxamide propylene glycol NB-2OH (2)
[0074] Take the three-neck round bottom bottle cooled after high temperature treatment, add cis-5-norbornene-exo-2,3-dicarboxylic acid anhydride (1g, 6.1mmol) and aminomethylpropanediol (0.96g, 9.15mmol), and add 30mL of toluene. Connect the h-type water separator at the mouth of the bottle, connect the serpentine condenser above it, and reflux the reaction in an oil bath at 135°C for 16 hours. After the reaction, the toluene is distilled off under re...
Embodiment 3
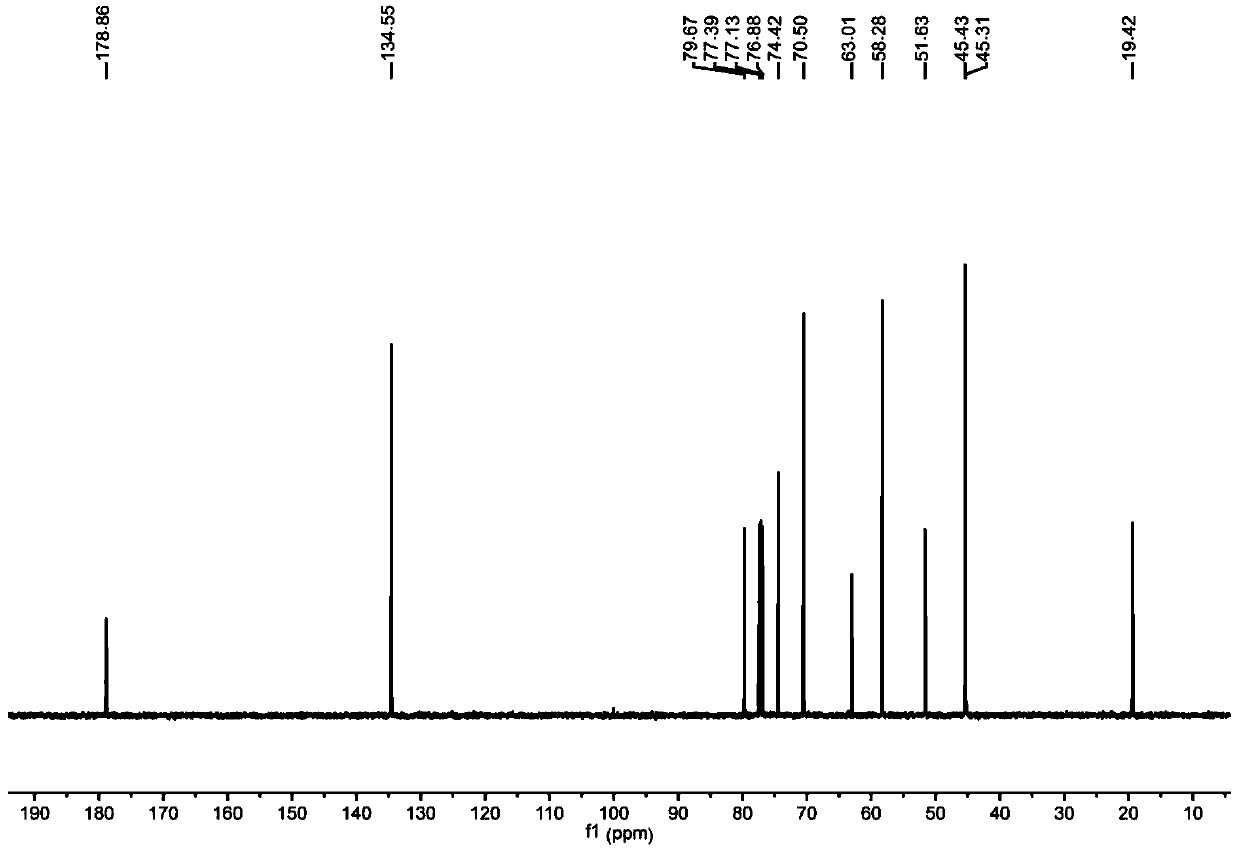
[0086] In this example, alkyne-terminated norbornene derivatives and azide-containing sugar compounds were used as raw materials to synthesize and prepare by copper-catalyzed azide-terminal alkyne [3+2] cycloaddition (CuAAC) reaction. NB-2Man-OAc and NB-2Gal-OAc two types of sugar-containing polymer monomers with protective groups, followed by ring-opening metathesis polymerization (ROMP) to prepare block copolymers P((( NB-2Man-OH)-b-(NB-2Gal-OH)).
[0087] Step 1 and Step 2 are the same as in Example 1.
[0088] 3. Synthesis of NB-2Man-OAc (M1) containing bilateral α-D-mannose norbornene derivative monomer
[0089]Add 2,2-diyne-norbornenedicarboxamide diethyl ether (1.465 g, 4.5 mmol) and acetyl-protected α-D-mannose azide (1.4 g, 3.75 mmol) into a dry reaction flask ), and tert-butanol (8 mL) and deionized water (4 mL) were added. Then copper sulfate pentahydrate (0.5625g, 2.25mmol) and sodium ascorbate (0.74g, 3.75mmol) were added, nitrogen gas was introduced, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com