Method for preparing optically active (S)-bufuralol by enzyme catalysis
A technology for catalytic preparation and optical activity, applied in the field of chiral compound synthesis, can solve the problems of low yield and optical purity, and achieve the effects of high optical purity of products, easy availability of raw materials and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
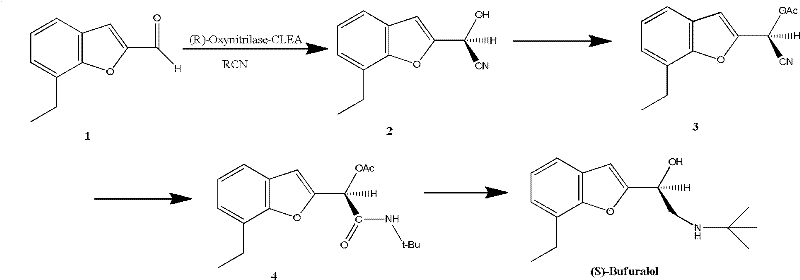
[0033] The preparation of (S)-bufurolol catalyzed by the cross-linking enzyme polymer of almond nitrilase comprises the following steps:
[0034] (1) Preparation of almond nitrilase cross-linked enzyme polymer
[0035] Soak peach kernels with 0.1% (v / v) sodium hypochlorite aqueous solution for 3 hours, rinse with deionized water, dry, add 3% polyvinylpyrrolidone and potassium phosphate buffer (10mM, pH6.0, 100mL / 100mg) and homogenize Homogenize, filter, centrifuge the filtrate, and collect the supernatant. The supernatant is precipitated with ammonium sulfate at 0-30%, 30-80%, and the 30-80% precipitate is centrifuged, and the above-mentioned potassium phosphate buffer solution is used to dissolve the precipitate, dialyzed overnight, centrifuged, and the supernatant is collected. The above enzyme purification processes were all carried out at 4°C. Add 80% (v / v) ethylene glycol dimethyl ether to the supernatant at 0°C to precipitate the enzyme protein. Add 0.5% (v / v) glutara...
Embodiment 2
[0055] The preparation of (S)-bufurolol catalyzed by the cross-linking enzyme polymer of almond nitrilase comprises the following steps:
[0056] (1) Preparation of almond nitrilase cross-linked enzyme polymer
[0057] The preparation method is described in step (1) in Example 1.
[0058] (2) synthesis of formula 2 compound
[0059]
[0060] Take 13mmol, 600mg of almond alcohol nitrilase cross-linking enzyme polymer, add 0.75mL of citric acid buffer (pH value is 5.0, 0.15mol / L) and 15mL of methyl tert-butyl ether, 8mmol of acetonitrile alcohol, 30 ℃ constant temperature conditions After reacting for 48 hours, the reaction liquid was filtered and concentrated under reduced pressure to obtain product 2.
[0061] (3) synthesis of formula 3 compounds
[0062]
[0063] Add 5 mL of CH to 2 2 Cl 2 Dissolve, acetyl chloride 6mmol, 0.06mmol PPY, react at room temperature for 1.5 hours, add 25mL CH 2 Cl 2 and 25 mL saturated NaHCO 3 solution, the aqueous phase was then CH ...
Embodiment 3
[0077] Lilinol nitrilase cross-linking enzyme polymer catalyzes the preparation of (S)-bufurolol, and the specific steps include:
[0078] (1) Preparation of linalonitrile enzyme cross-linked enzyme polymer
[0079] The preparation method is as described in step (1) in Example 1.
[0080] (2) synthesis of formula 2 compound
[0081]
[0082] Take 13mmol, 1000mg of linaloyl nitrilase cross-linking enzyme polymer, add 1.5mL of citric acid buffer (pH value is 5.5, 0.2mol / L) and 15mL of diisopropyl ether, HCN12mmol, and react for 24 hours under constant temperature at 40°C Afterwards, the reaction solution was filtered and concentrated under reduced pressure to obtain product 2.
[0083] (3) synthesis of formula 3 compounds
[0084]
[0085] Add 5 mL of CH to 2 2 Cl 2 Dissolve, 9mmol acetic acid, 0.3mmol DMAP, react at room temperature for 1 hour, add 25mL CH to the reaction solution 2 Cl 2 and 25 mL saturated NaHCO 3 solution, the aqueous phase was then CH 2 Cl 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com