Preparation method for disubstituted styrene derivative
A technology of styrene derivatives and styrenes, applied in the direction of organic chemistry, etc., can solve the problems of low yield, cumbersome steps of disubstituted styrene derivatives, environmental pollution, etc., and achieves high research value, mild conditions, and methods. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
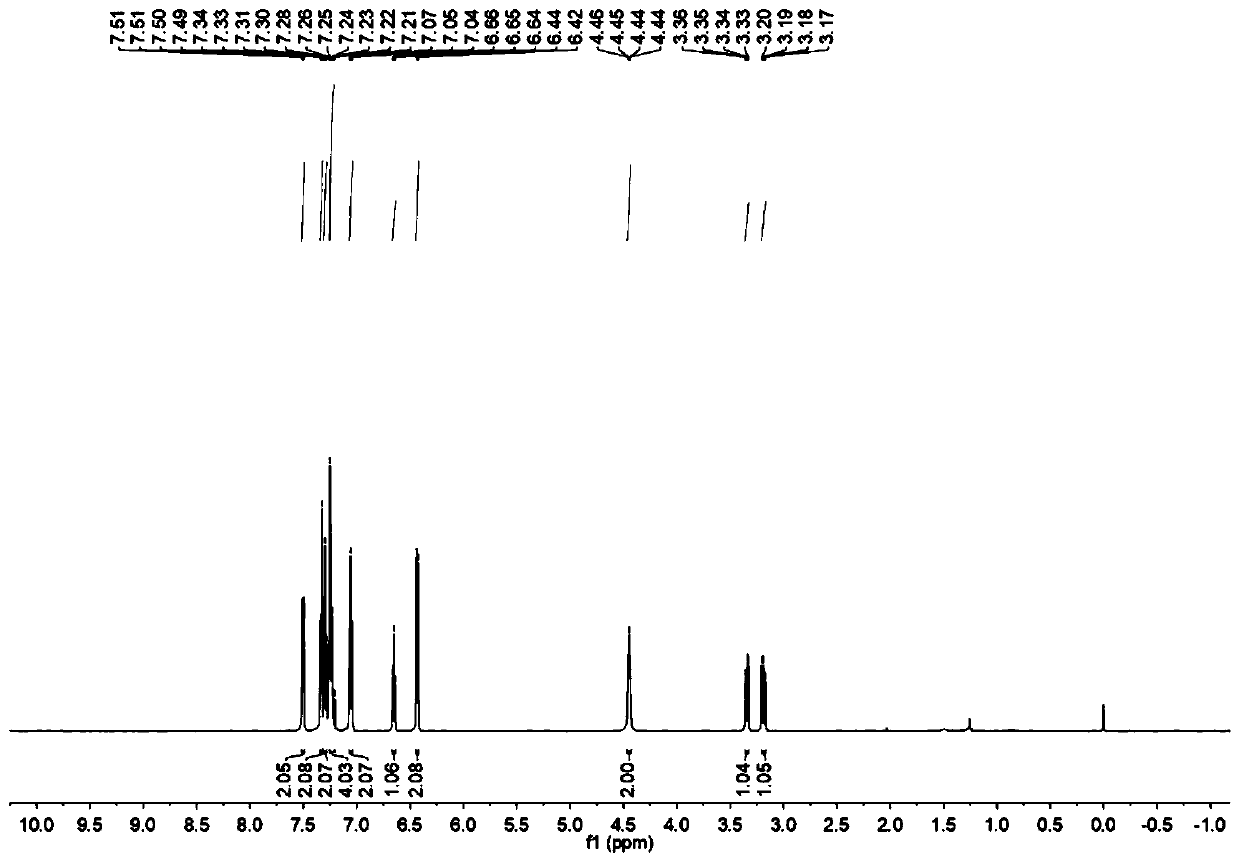
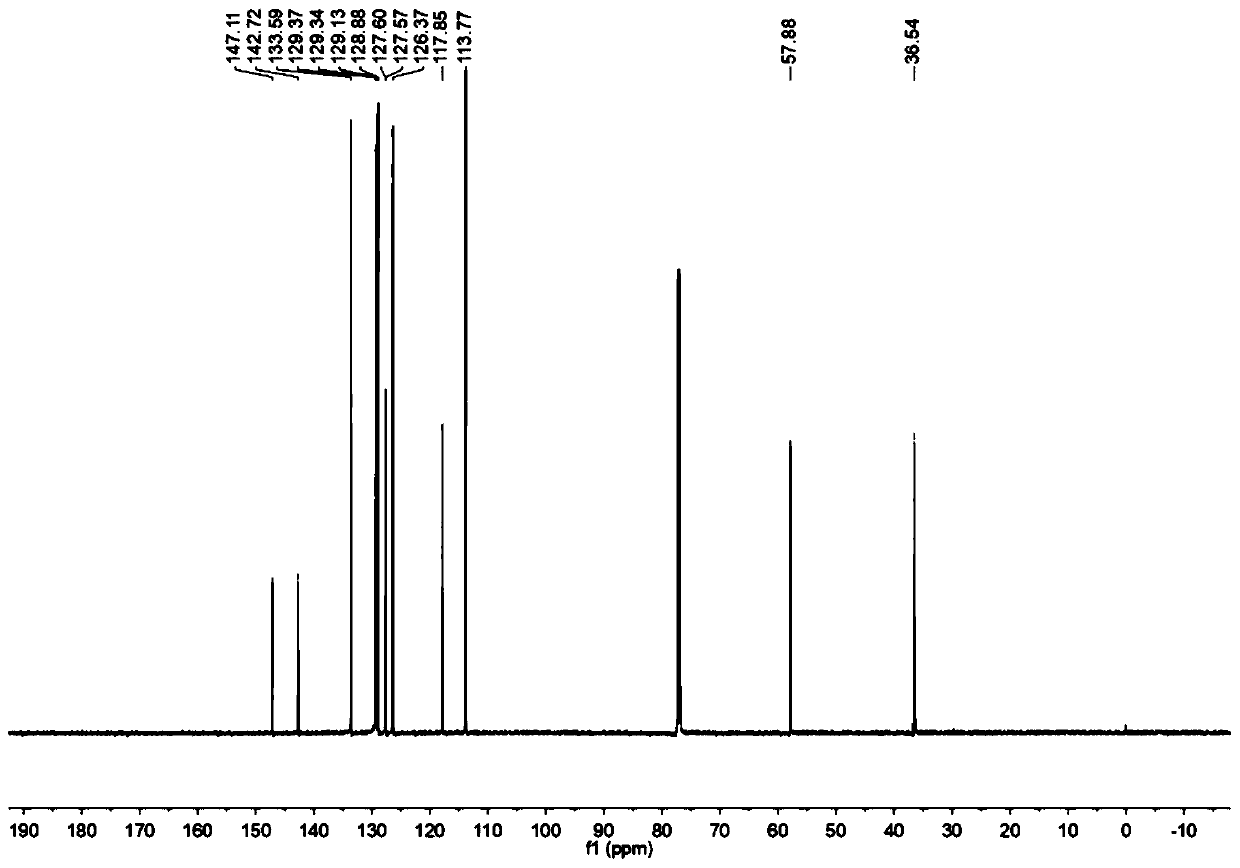
[0013] Specific embodiment one: the preparation method of a kind of disubstituted styrene derivatives in this embodiment comprises the following steps:
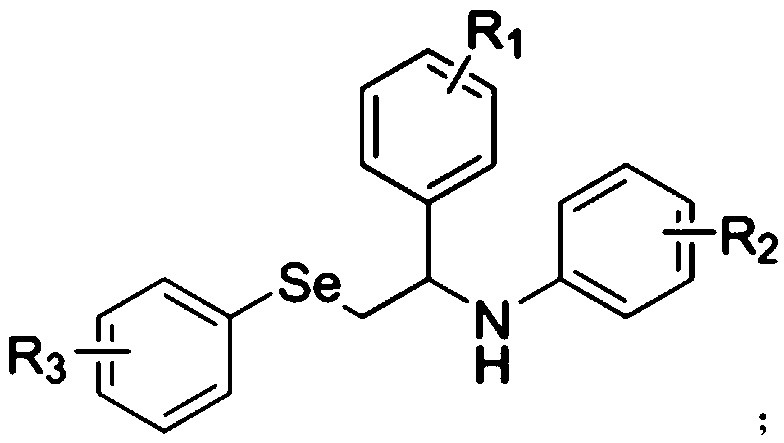
[0014] At room temperature, under a nitrogen or oxygen atmosphere, dissolve styrene derivatives, aniline compounds, diphenyldiselenide compounds and photocatalysts in organic solvents, mix well, and place them under blue LEDs for reaction. After the end, spin the solvent, and then separate and purify through silica gel column chromatography to obtain disubstituted styrene derivatives; wherein the molar ratio of styrene compounds, aniline compounds, diphenyldiselenide compounds and photocatalysts is 1:1.5:0.6:0.1; wherein the chemical structural formula of styrene derivatives is:
[0015] The chemical structural formula of aniline compounds is:
[0016] The chemical structural formula of diphenyl diselenide compound is:
[0017] Among them, R 1 , R 2 and R 3 is halogen, alkoxy or alkyl.
[0018] The structural form...
specific Embodiment approach 2
[0024] Specific embodiment two: the difference between this embodiment and specific embodiment one is: styrene derivatives are styrene, 4-methoxystyrene, 4-bromostyrene, 2-chlorostyrene or 2-methylbenzene vinyl. Others are the same as the first embodiment.
specific Embodiment approach 3
[0025] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that the aniline compound is 3-methylaniline, 2-tert-butylaniline, 3-nitroaniline, N-methylaniline or Benzotriazole. Others are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com