Ultra-fine boron carbide polycrystalline powder prepared through organic boron-containing precursor self-propagating method
A precursor, self-propagating technology, applied in the field of boron carbide material preparation, can solve the problems of high cost, low purity of boron carbide ultrafine powder, and difficulty of ultrafine boron carbide, and achieves low preparation cost and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take 10.03 grams of PVA (the degree of polymerization is 1800-2000, and the degree of alcoholysis is not less than 98%), 6.98 grams of boric acid, and 40.07 grams of ethanol (99.5%) are put into a glass bottle, stirred at 50°C for 5 hours, and then heated at 250- Spray drying at 300° C. to obtain 12.93 g of PVA-boric acid precursor powder.
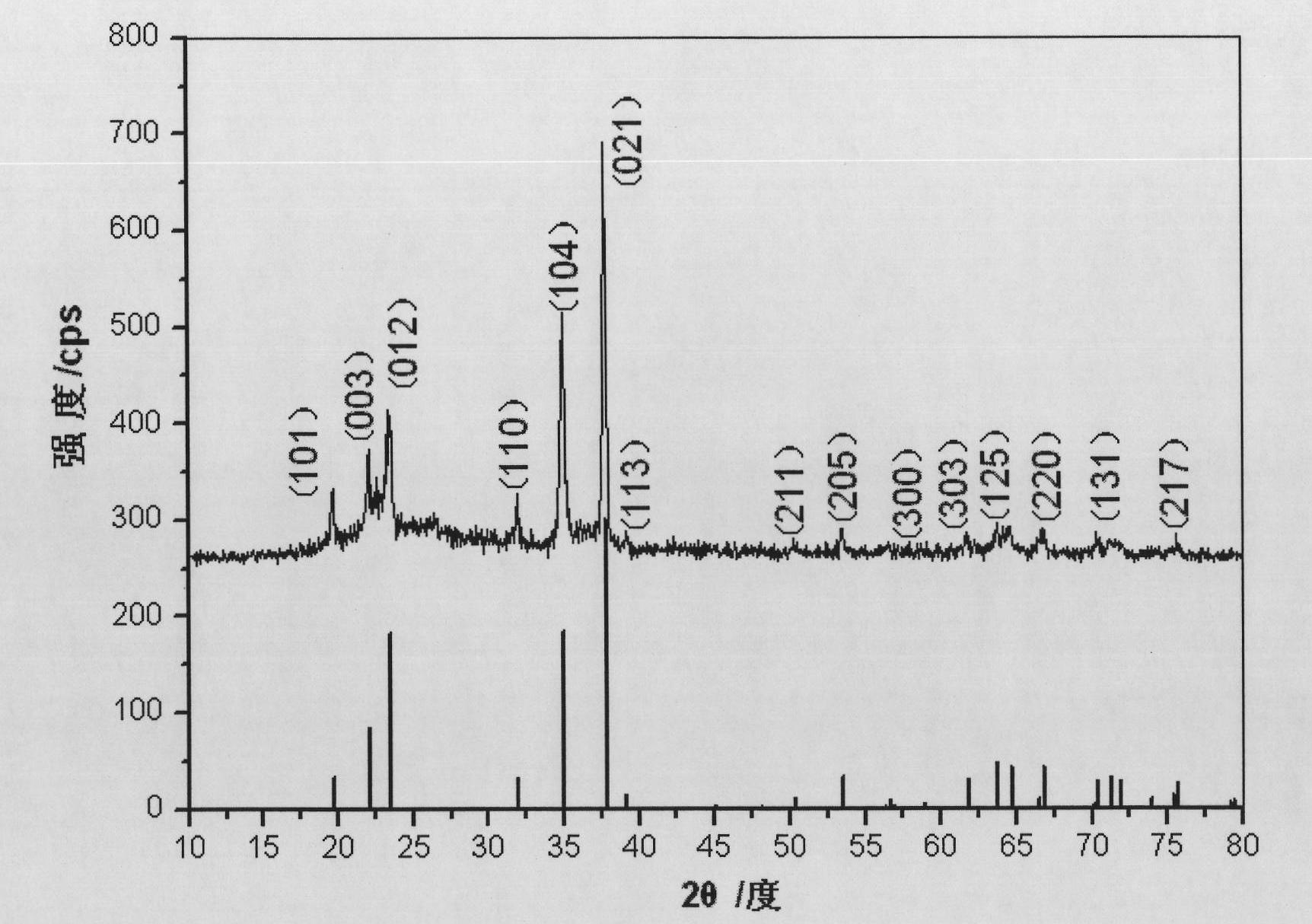
[0040] Mix PVA-boric acid precursor powder, 45.26 grams of boric anhydride, and 49.13 grams of magnesium powder (mass ratio: 1:3.5:3.8, particle size 100-300 mesh powder), then briquetting, and then energize in argon at normal temperature and pressure The hot tungsten wire was ignited and self-propagating combustion occurred, and the furnace was cooled to room temperature to obtain 58.29 grams of combustion reaction products. figure 1 It is a photo of the combustion product obtained by self-propagating reaction after the PVA-boric acid precursor is mixed with boric anhydride and magnesium powder.
[0041] Put the combustion reactio...
Embodiment 2
[0047] Take 10.09 grams of PVA (the degree of polymerization is 1800-2000, the degree of alcoholysis is not less than 98%), 7.06 grams of boric acid, 50.07 grams of ethanol (99.5%) are put into a glass reactor, stirred at 50°C for 5 hours, and then heated at 250 Spray drying at ~300°C to obtain 13.04 g of PVA-boric acid precursor powder.
[0048] PVA-boric acid precursor powder, 46.94 grams of boric anhydride, 54.77 grams of magnesium powder (mass ratio 1:3.6:4.2, particle size 100-300 mesh powder) were mixed and placed in a stainless steel reactor, and ignited at 800 ° C. Propagate the reaction and cool to room temperature with the furnace to obtain 58.79 g of combustion reaction products.
[0049] Take the combustion product and put it into excess 35-38wt.% concentrated hydrochloric acid, stir and soak at 50-70°C for 12 hours, then suction filter, wash the filter cake with distilled water until neutral, and dry the washed filter cake at 80°C for 24 hours. Hour, obtain 12.65...
Embodiment 3
[0052] Take 10.01 grams of PVA (the degree of polymerization is 1800-2000, and the degree of alcoholysis is not less than 98%), 7.11 grams of boric acid, and 39.9 grams of ethanol (99.5%) are put into a glass reactor, stirred at 80°C for 5 hours, and then heated at 250 Spray drying at ~300°C to obtain 12.94 g of PVA-boric acid precursor powder.
[0053] Mix PVA-boric acid precursor powder, 45.94 grams of boric anhydride, and 51.76 grams of magnesium powder (mass ratio: 1:3.55:4.0, powder with a particle size of 100 to 300 meshes) and briquette them, then electrify them in argon gas at normal temperature and pressure The self-propagating reaction was ignited by the tungsten wire, cooled to room temperature with the furnace, and 59.34 grams of combustion reaction products were obtained.
[0054] Put the self-propagating reaction product into excess 35-38% concentrated hydrochloric acid, stir and soak at 50-70°C for 12 hours, then filter with suction, wash the filter cake with di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com