Zinc-polyaniline cell and preparation method thereof
A polyaniline battery and polyaniline technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of high cost, low specific energy, and short life of rechargeable batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
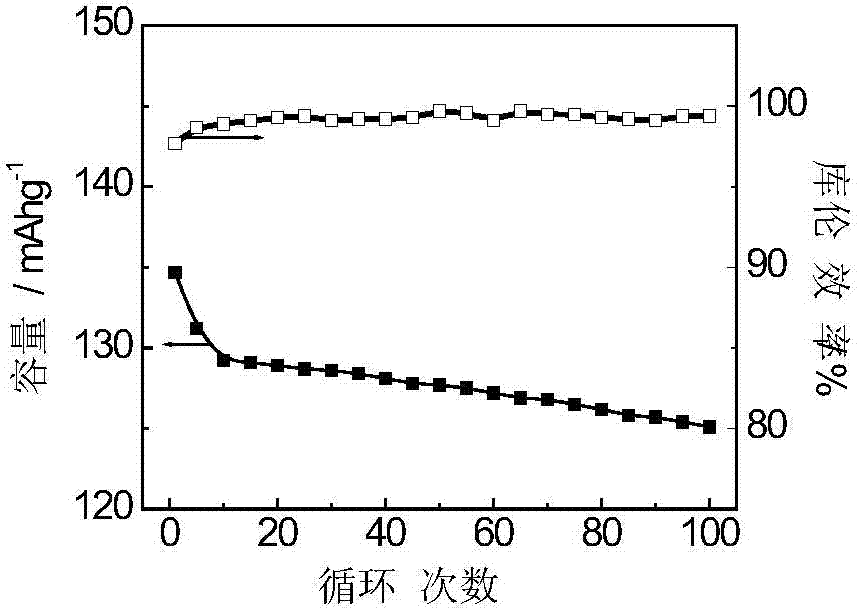
Embodiment 1
[0036] The new synthesis process of adding activated carbon and two oxidants can make the yield and capacity of polyaniline in the whole synthesis process very high, which is the key indicator of future industrialization.
[0037] The production process of polyaniline synthesis is as follows: take 25L concentration as 0.5molL -1 After cooling down to below 5°C, add a certain amount of aniline (optimally 1.4kg) and react for 5 minutes; add an appropriate amount of activated carbon (optimally 170g), stir and disperse for 30min, then cool down in an ice-water bath to 0°C, then add 190 g of manganese dioxide, and react for 10 minutes. Take another 10L of hydrochloric acid of a certain concentration (1.0molL -1 ), add ammonium persulfate (optimally 3.8kg), and stir to dissolve. Under the condition of stirring, the ammonium persulfate solution was added dropwise, and the drop was controlled within 2 hours. After the dropwise addition, the stirring was continued, and the reaction w...
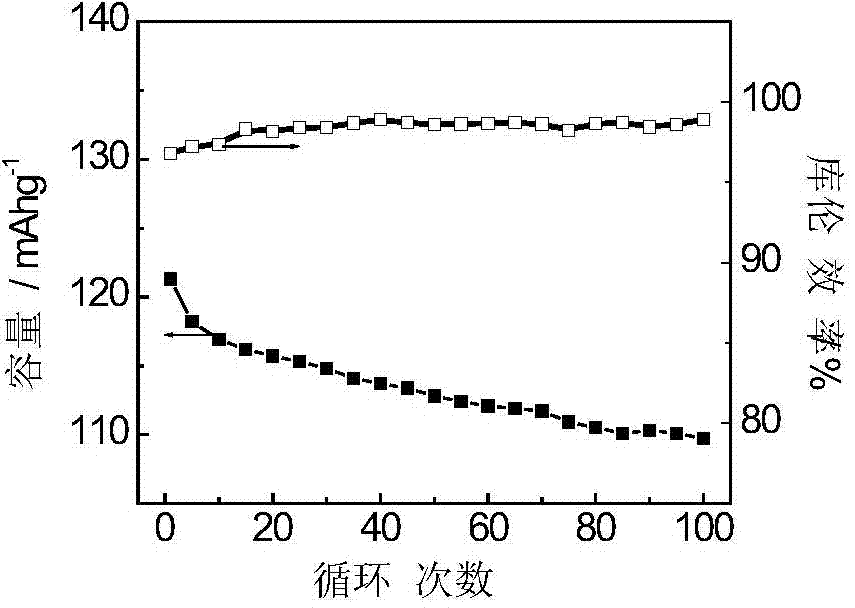
Embodiment 2
[0041] The production process of polyaniline synthesis is as follows: take 25L concentration as 1molL -1 Hydrochloric acid, after cooling down to below 5°C, add a certain amount of aniline (optimally 1.4kg) while stirring, and react for (how many minutes) min; add an appropriate amount of activated carbon (optimally 170g), stir and disperse for 30min, Cool down to 0°C in an ice-water bath, then add 190 g of manganese dioxide, and react for 10 minutes. Take another 10L of hydrochloric acid of a certain concentration (1.0molL -1 ), add potassium persulfate (optimally 3.8kg), and stir to dissolve. Under the condition of stirring, the potassium persulfate solution is added dropwise, and the drop is controlled within 2 hours. After the drop is completed, continue to stir and react for another 4 hours. The temperature of the entire reaction process is controlled at 5°C. After the reaction, wash with deionized water repeatedly 5 times, and then use 0.1molL -1 HCl was replaced for ...
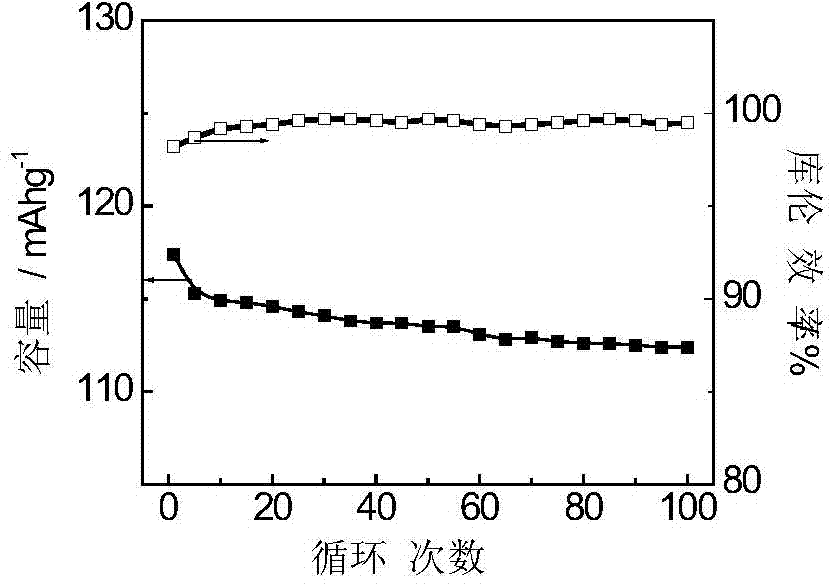
Embodiment 3
[0044] The production process of polyaniline synthesis is as follows: take 25L concentration as 3molL -1 After cooling down to below 5°C, add a certain amount of aniline (optimally 1.4kg) and react for 5 minutes; add an appropriate amount of activated carbon (optimally 170g), stir and disperse for 30min, then cool down in an ice-water bath to 0°C, then add 190 g of manganese dioxide, and react for 10 minutes. Take another 10L of hydrochloric acid of a certain concentration (1.0molL -1 ), add sodium persulfate (optimally 3.8kg), and stir to dissolve. Under the condition of stirring, the sodium persulfate solution is added dropwise, and the drop is controlled within 2 hours. After the drop is completed, continue to stir and react for another 4 hours. The temperature of the whole reaction process is controlled at 5°C. After the reaction, wash with deionized water repeatedly 5 times, and then use 0.1molL -1 HCl was replaced for 1 h, washed with water for 3 times, and then repla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com