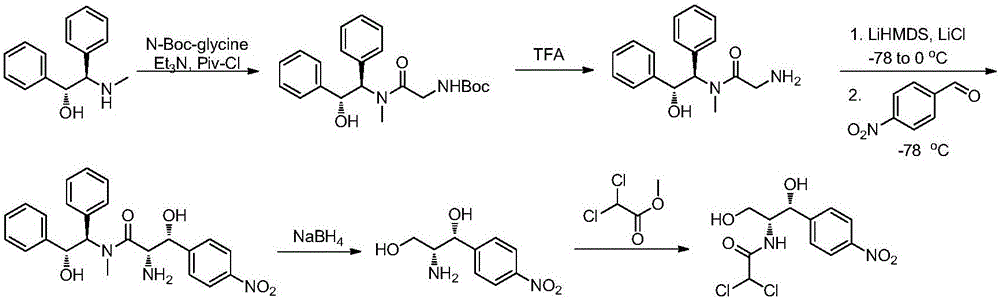
Preparation method of chloramphenicol compounds
A technology of chloramphenicol and compounds, which is applied in the fields of biopharmaceuticals and biochemical industries, can solve the problems of harsh reaction conditions, low atom economy, unfriendly environment, etc., and achieve the effects of simple operation, simple post-treatment, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9
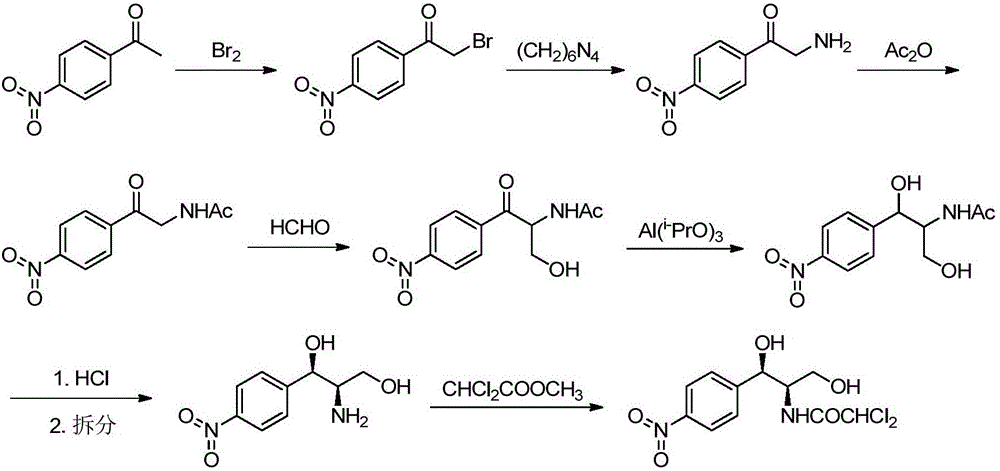
[0046] Example 9 is the preparation of (R, R)-thrao-type-1-p-nitrophenyl-2-amino-1,3-propanediol (D-amino compound) Example 10 is a method for preparing chloramphenicol using the D-amino compound prepared in Example 9, and the synthetic route is:
[0047]
Embodiment 1
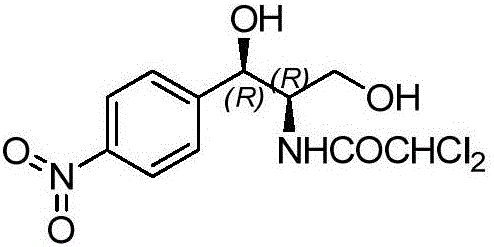
[0051] The structure of the chloramphenicol intermediate used in this embodiment is as follows:
[0052]
[0053] Take 1g of chloramphenicol intermediate and 1.25g of glucose in a 100mL three-necked flask, and then add 50mL of PBS buffer with a pH of 6.5 and a concentration of 0.05mol / L; put the three-necked flask into the reaction pot, Set the speed to 850rpm, the temperature is 30℃, and then add 5mg NADP respectively + , 20mg glucose dehydrogenase (purchased from Suzhou Yinhang Biological Technology Co., Ltd., the product number is YH1901, only one of the models is given here to illustrate the effect of the present invention, and the various types of products on the market are useful for realizing the present invention. There is no difference in terms of the purpose, the same below, no further details will be given below), and 30mg ketoreductase powder (purchased from Suzhou Yinhang Biotechnology Co., Ltd.) to obtain a mixed solution, and the temperature of the mixed solution is...
Embodiment 2
[0060] The structure of the chloramphenicol intermediate used in this embodiment is as follows:
[0061]
[0062] Take 5g of chloramphenicol intermediate and 6.25g of glucose in a 100mL three-necked flask, add 50mL of PBS buffer with a pH of 6.5 and a concentration of 0.05mol / L; put the three-necked flask into the reaction pot, set the speed at 850rpm and temperature 30℃, then add 5mg NADP + , 100mg glucose dehydrogenase, and 100mg ketoreductase powder (purchased from Suzhou Yinhang Biotechnology Co., Ltd., the product number is YH2047, only one of the models is given here to illustrate the effect of the present invention, and each model is commercially available There is no difference between the products for achieving the purpose of the present invention, the same below, and will not be repeated hereafter), to obtain a mixed solution, and make the mixed solution react at a temperature of 30°C, and at the same time, use 2mol / L of NaOH solution to maintain the pH value of the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com