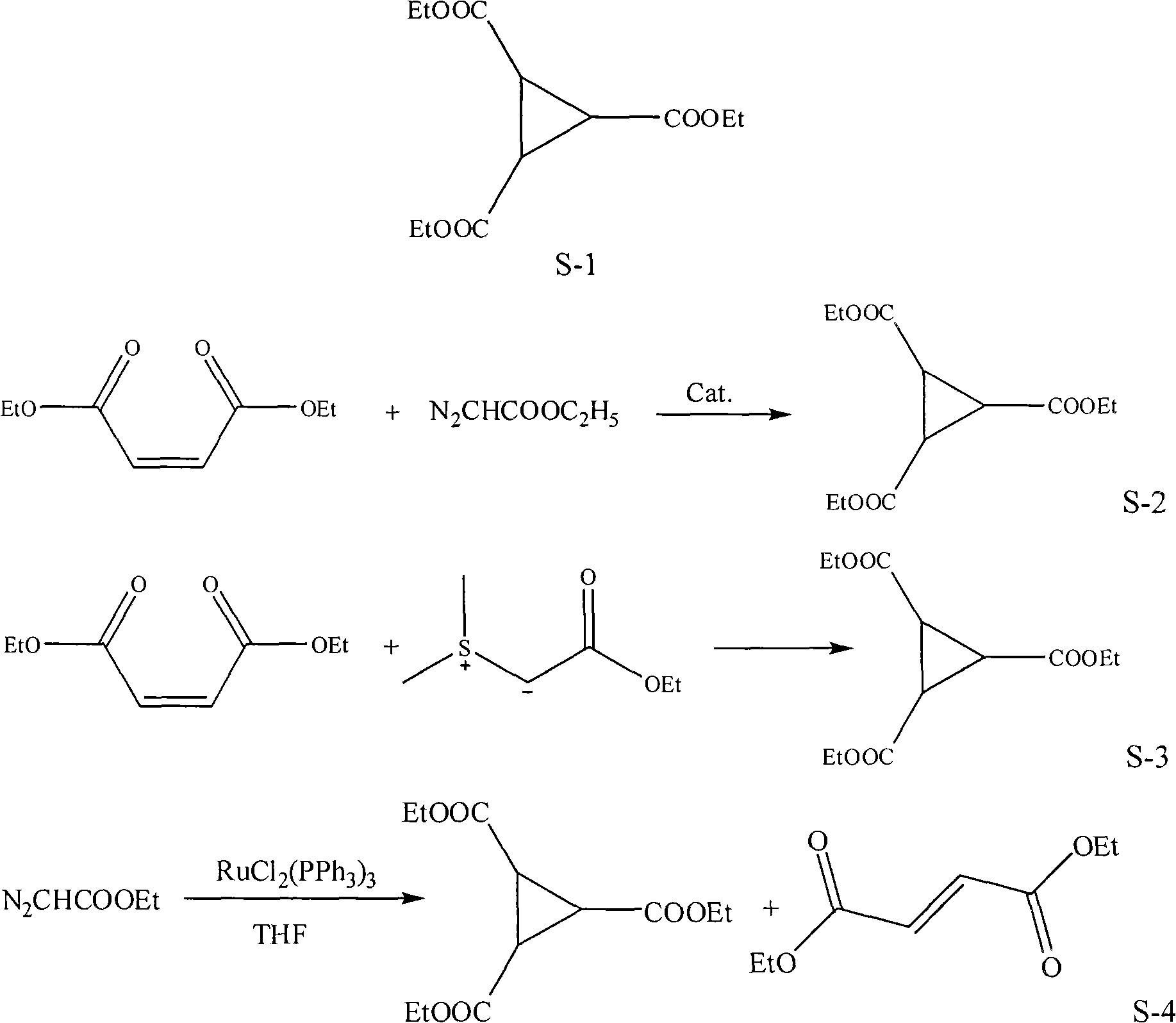
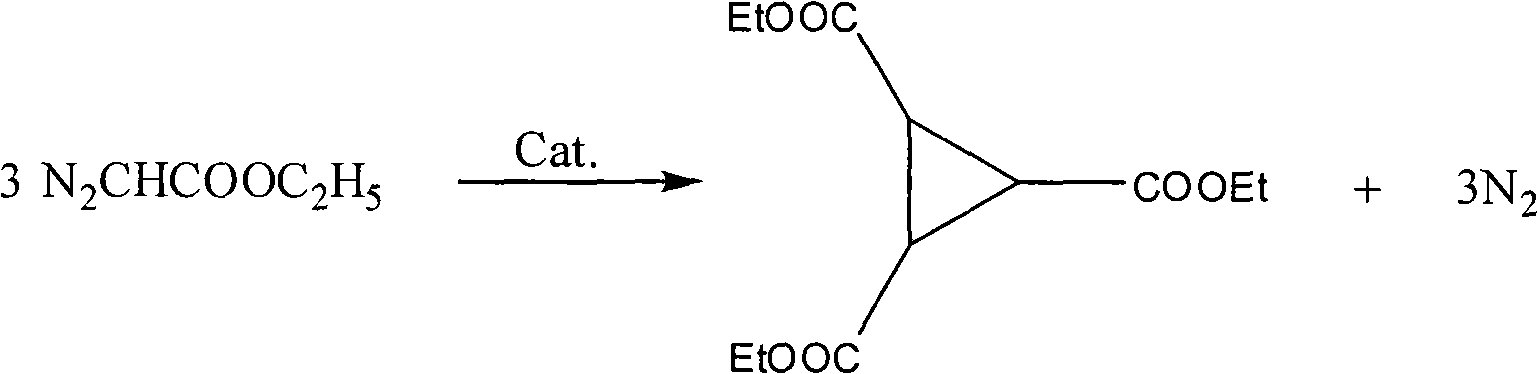
Preparation method of 1,2,3-cyclopropyl tricarboxylate
A technology of cyclopropanetricarboxylic acid and triethyl ester, which is applied in the field 1, can solve problems such as difficult preparation, high price, and environmental pollution, and achieve the effect of low cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, a kind of preparation method of 1,2,3-triethyl cyclopropanetricarboxylate, carries out following steps successively:
[0017] Step 1), prepare ethyl diazoacetate:
[0018] Put 14.0g (0.1mol) glycine ethyl ester hydrochloride, 20ml water, 1ml mass concentration 98% concentrated sulfuric acid, and 50ml dichloroethane into a 250ml flask, cool to 10°C, and add 25ml nitrous acid dropwise under stirring Aqueous sodium solution (27.5%, 0.11 mol), dripped over 20 minutes, and stopped the reaction after stirring for 30 minutes. Separation, the aqueous phase was extracted with dichloroethane (2 × 20ml), the combined organic phase was washed with saturated sodium bicarbonate solution (2 × 10ml), dried over anhydrous magnesium sulfate (refer to "Fine Chemical Intermediates" 2008, 38 (1): 40~43), obtain the dichloroethane solution 118.0g of ethyl diazoacetate (GC analysis ethyl diazoacetate content is 9.1%, equivalent to pure ethyl diazoacetate 10.7g), the weight of g...
Embodiment 2~8
[0022] Step 1) is the same as in Example 1.
[0023] Change the following reaction conditions in the step 2) of embodiment 1:
[0024] Step 2) in organic solvent (being called for short S), the quality of organic solvent and ethyl diazoacetate mass ratio (being called for short R in step 2) 1 ), catalyst (abbreviated as C) in step 2, the quality of catalyst in step 2 and ethyl diazoacetate mass ratio (abbreviated as R 2 ), temperature of reaction (abbreviated T) in step 2, reaction time (abbreviated t) in step 2, dripping ethyl diazoacetate time (abbreviated t) in step 2 1 ), continue the reaction time in step 2 (referred to as t 2 ), t=t 1 +t 2 , to obtain Examples 2-8, thereby obtaining the corresponding yield of 1,2,3-triethyl cyclopropanetricarboxylate (abbreviated as Y). See Table 1 for details and data results.
[0025] Table 1
[0026] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com