A method for preparing trans-D-chrysanthemic acid
A technology of trans-D-chrysanthemum acid and permethrin, which is applied in the direction of carboxylate preparation, carboxylate preparation, organic chemical methods, etc., can solve the problems of harsh reaction conditions, low reactivity, and high cost of ligands, and achieve synthesis Simple, high stereoselectivity, good reactivity
Active Publication Date: 2022-05-31
DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, these systems have problems such as low reactivity, harsh reaction conditions, and high cost of ligands. Therefore, it is of great significance to develop a low-cost, high-activity, high-stereoselective asymmetric cyclopropanation method for ligand synthesis.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0039]
[0043]
Embodiment 2
Embodiment 3
[0049] K
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
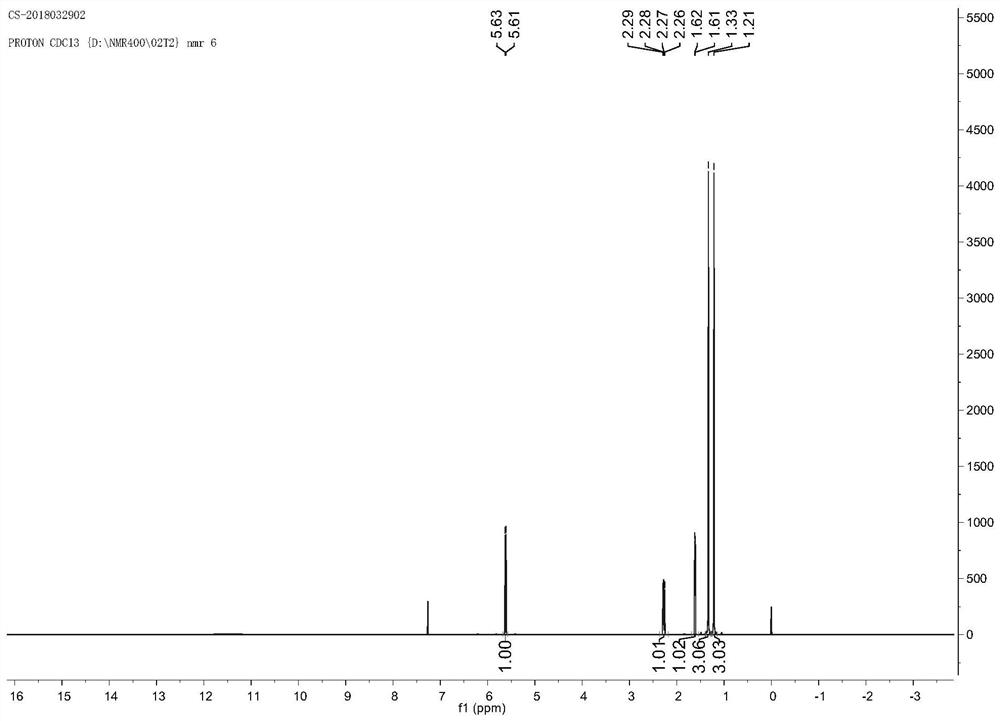
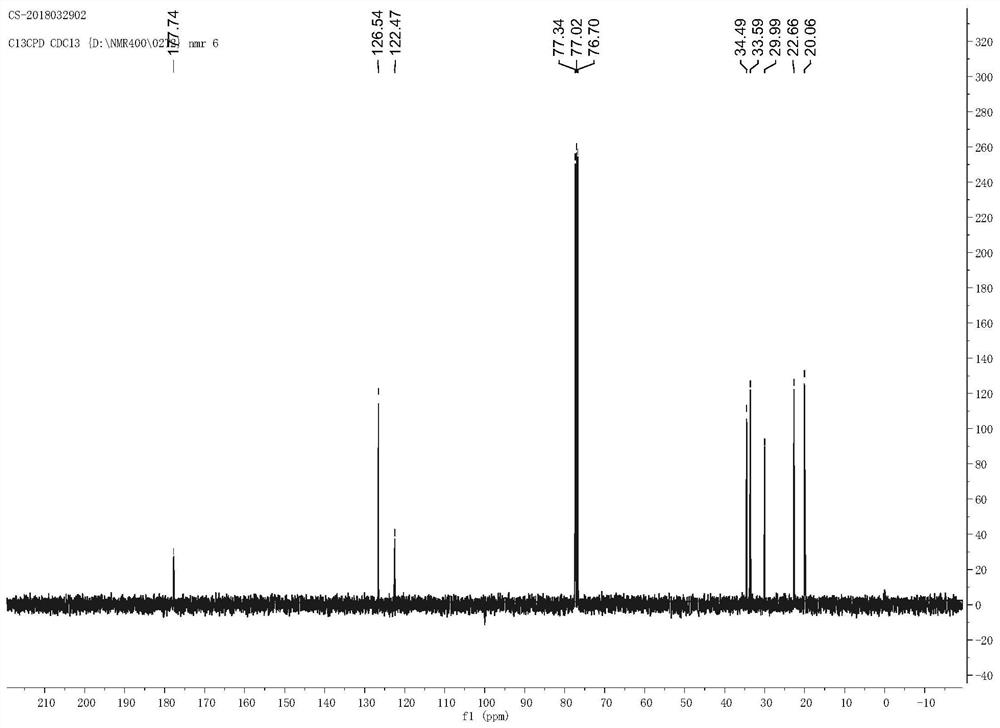
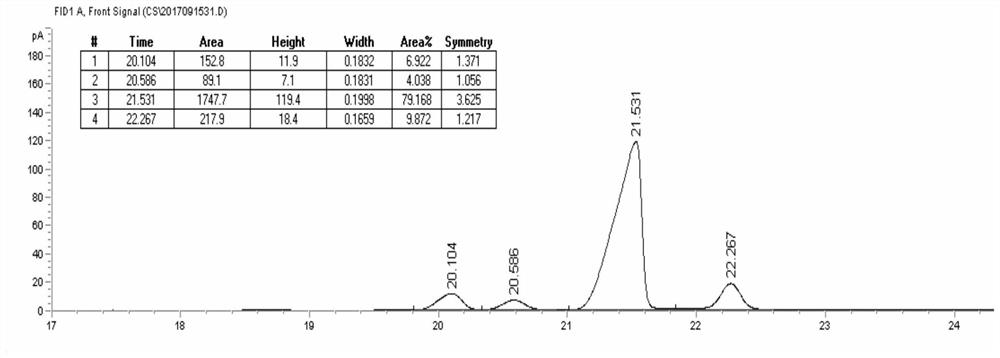
The invention provides a method for preparing trans-D-chrysanthemic acid, which belongs to the field of organic synthesis. The method of the present invention is that 2-methyl-5,5,5-trichloro-2-pentene and ethyl diazoacetate are synthesized by an asymmetric cyclopropanation reaction to trans-derothrin, and then hydrolyzed to generate trans- D-V-chrysanthemic acid (DV-chrysanthemic acid). The chiral ruthenium catalyst used is generated in situ by ruthenium salts and chiral tridentate P,N,N-ligands in various polar and non-polar solvents. The present invention can conveniently synthesize trans D-chrysanthemic acid (DV-chrysanthemic acid), and its enantiomeric excess percentage is as high as 90%. The invention has the characteristics of simple operation, readily available raw materials, wide application range of substrates, high enantioselectivity and the like.
Description
A kind of method for preparing trans-dextrinic acid technical field The invention belongs to the field of organic synthesis, be specifically related to a kind of method of preparing trans-dextrinic acid. Background technique Trans-D-Chrysanthemum acid (DV chrysanthemic acid) is an important organic synthesis intermediate, can be used for the preparation of various bioactive natural and unnatural compounds. In recent years, organic chemists at home and abroad have synthesized chiral ligands with different frameworks to control A lot of work has been done on the preparation of chiral chrysanthemic acid by asymmetric cyclopropanation, and great success has been achieved. As early as 1966, Nozaki catalyzed styrene and diazoacetic acid through the catalyst formed by Schiff base and transition metal. Asymmetric cyclopropanation of esters yielding ee values of 10% (cis‑) and 6% (trans‑). The reaction tells people: Transition Chiral ligands in metal complexes can induc...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C51/347C07C61/40
CPCC07C67/343C07C51/09C07C51/347C07B2200/07C07C2601/02C07C69/74C07C61/40Y02P20/584
Inventor 胡向平陈松
Owner DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com