A kind of method for preparing n-sulfonimide
A technology of sulfonimide and sulfonamide, which is applied in the field of preparing N-sulfonimide, can solve problems such as unsuitability, and achieve the effects of less reaction steps, high atom economy, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
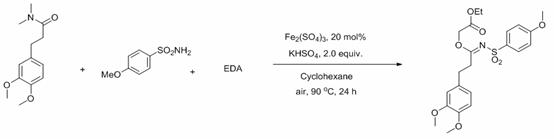
[0026]
[0027] To the tube was added amide (0.5 mmol), p-methoxybenzenesulfonamide (0.75 mmol), iron sulfate (20 % mmol), potassium bisulfate (2.0 eq), then cyclohexane (2 mL), and finally Ethyl diazoacetate EDA (3.0 mmol), and the mixture was reacted in an oil bath at 90°C for 24 hours under an air atmosphere. After the reaction was completed, it was quenched with saturated sodium chloride solution, extracted with ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, and the solvent was spin-dried under reduced pressure. The product can be obtained by column chromatography with a mixed solvent of ethyl acetate and petroleum ether with a yield of 90%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthetic product is consistent with the theoretical analysis.
[0028] 1 H NMR (400 MHz, CDCl 3 ) δ 7.80–7.75 (m, 2H), 6.96–6.89 (m, 2H), 4.58(s, 2H), 4.05 (q, J = 7.1 Hz, 2H), 3...
Embodiment 2
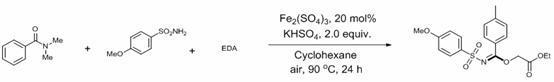
[0030] On the basis of Example 1, the reaction conditions were changed by a single factor:
[0031] Without adding ferric sulfate, yield: 68%;
[0032] Without potassium hydrogen sulfate, yield: 35%;
[0033] Replace ferric sulfate with cobalt sulfate, yield: 67%;
[0034] Replace iron sulfate with cobalt acetylacetonate, yield: 32%;
[0035] Replace ferric sulfate with ferric acetylacetonate, yield: 67%;
[0036] Replace ferric sulfate with ferrous sulfate, yield: 70%;
[0037] Replace ferric sulfate with ferric oxide, yield: 65%;
[0038] Replace ferric sulfate with copper acetate, yield: 35%;
[0039] Replace potassium hydrogen sulfate with potassium dihydrogen phosphate, yield: 33%;
[0040] Replace potassium hydrogen sulfate with sodium dihydrogen phosphate, yield: 23%;
[0041] Replace cyclohexane with n-hexane, yield: 22%;
[0042] Replace cyclohexane with 1,4-dioxane, yield: 32%;
[0043] Replace cyclohexane with acetonitrile, yield: 38%;
[0044] Replace cyc...
Embodiment 3
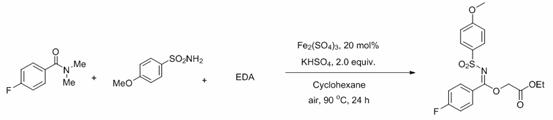
[0048]
[0049]To the tube was added amide (0.5 mmol), sulfonamide (0.75 mmol), iron sulfate (20 % mmol), potassium bisulfate (2.0 eq), then cyclohexane (2 mL) and finally ethyl diazoacetate EDA (3.0 mmol), the mixture was reacted in an oil bath at 90 °C for 24 hours under an air atmosphere. After the reaction was completed, it was quenched with saturated sodium chloride solution, extracted with ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, and the solvent was spin-dried under reduced pressure. The product was obtained by column chromatography with a mixed solvent of ethyl acetate and petroleum ether with a yield of 66%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthetic product is consistent with the theoretical analysis.
[0050] 1 H NMR (400 MHz, CDCl 3 ) δ 7.82 (d, J = 8.1 Hz, 2H), 7.76 (d, J = 8.9Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 6.89 (d, J = 8.9 Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com