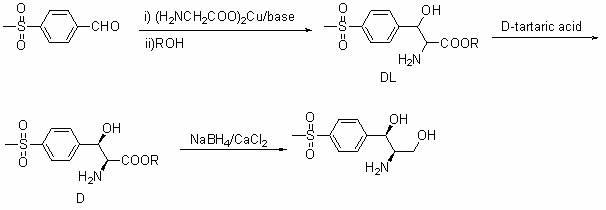
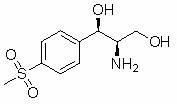
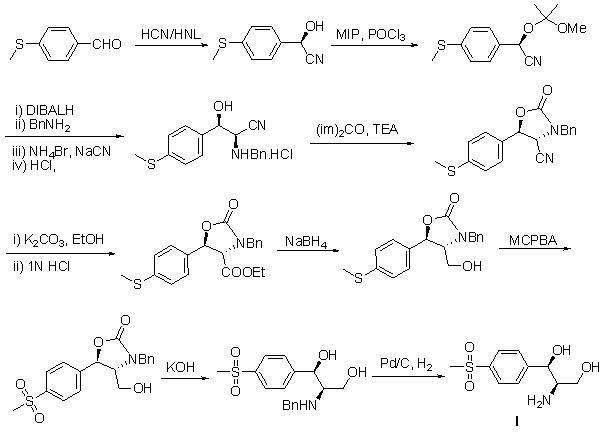
Method for synthesizing (1R, 2R)-1-p-methyl sulfone phenyl-2-amino-1,3-propanediol
A technology for the synthesis of thiamphenylphenyl and its method, which is applied in the field of synthesis of 1-p-thiamphenylphenyl-2-amino-1,3-propanediol (I), and can solve problems such as lengthy steps and large dosage , to achieve the effect of short route, easy operation and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 P-methylsulfonylbenzaldehyde (18.42 g, 0.10 mol), diphenylmethylamine (18.33 g, 0.10 mol), dichloroethane (200 mL), and anhydrous magnesium sulfate (40.00 g) were dried In the reaction flask, the reaction was carried out at room temperature for 12 hours. After the reaction is completed, filter and recover the solvent under reduced pressure to obtain a colorless oily substance, which is recrystallized with 95% ethanol, and dried to obtain a white crystal, which is compound (III) (9.60 g, 95%), mp: 139~140°C.
[0033] 1H NMR (CDCl 3 , ppm): δ=3.06 (s, 3H, CH 3 ), 5.67 (s, 1H, CH), 7.26 (m, 2H, ArH), 7.24-7.41 (m, 8H, ArH), 8.02 (m, 4H, ArH) 8.49 (s, 1H, NH).
[0034] ESI-MS: (m / z)=350.6 (M + ).
[0035] Two, (2 S , 3 S )-1-Diphenylmethyl-2-ethoxycarbonyl-3-p-sulfonylphenylacridine (IV).
Embodiment 21
[0036] Example 21 Under nitrogen protection, (2 R )-(+)-3,3'-diphenyl-[2,2'-binaphthalene]-1,1'-diol (4.39 g, 0.01 mol), triphenyl borate (11.60 g, 0.04 mol) was placed in a dry reaction flask, 50 ml of toluene was added, heated to 80 °C for 1 hour, and then the toluene was recovered under reduced pressure, kept at 80 °C under vacuum for 0.5 hours, cooled to room temperature, and dissolved in 50 ml of anhydrous toluene. It was added to a suspension of compound (III) (69.8 g, 0.20 mol) in anhydrous toluene (700 ml), stirred at room temperature for 5 minutes, cooled to -20°C, and added dropwise with ethyl diazoacetate (25.08 g, 0.22 mol). ) and continue to react for 6 hours. Then the solvent was recovered under reduced pressure, and the crude product was recrystallized twice with ethanol and water to obtain compound (IV) (59.52 g, 68.3%) as colorless crystals, mp: 122~123°C, optical rotation [α] 25 D = -20.9° (1.05, CHCl 3 ), the ee value is 99.7%.
[0037] 1 H NMR (CDCl ...
Embodiment 22
[0039] Example 22 Under nitrogen protection, (3 R )-(+)-2,2'-diphenyl-[3,3'-diphenanthrene]-4,4'-diol (5.38 g, 0.01 mol), triphenyl borate (11.60 g, 0.04 mol) ) was placed in a dry reaction flask, 50 ml of toluene was added, heated to 80 °C for 1 hour, the solvent was recovered under reduced pressure, and kept at 80 °C under vacuum for 0.5 hours, cooled to room temperature, dissolved in 50 ml of anhydrous toluene, and It was added to a suspension of compound (III) (69.8 g, 0.20 mol) in anhydrous toluene (700 ml), stirred at room temperature for 5 minutes, cooled to -20°C, and added dropwise with ethyl diazoacetate (25.08 g, 0.22 mol). ) and continue to react for 6 hours. Then the solvent was recovered under reduced pressure, and the crude product was recrystallized twice with ethanol and water to obtain compound (IV) (58.20 g, 66.8%) as colorless crystals. The melting point, optical rotation, 1 H NMR and MS were consistent with Example 21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com