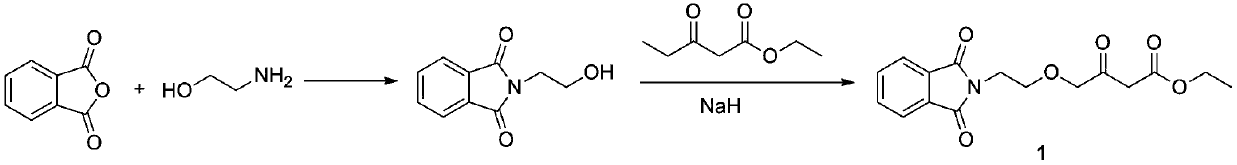
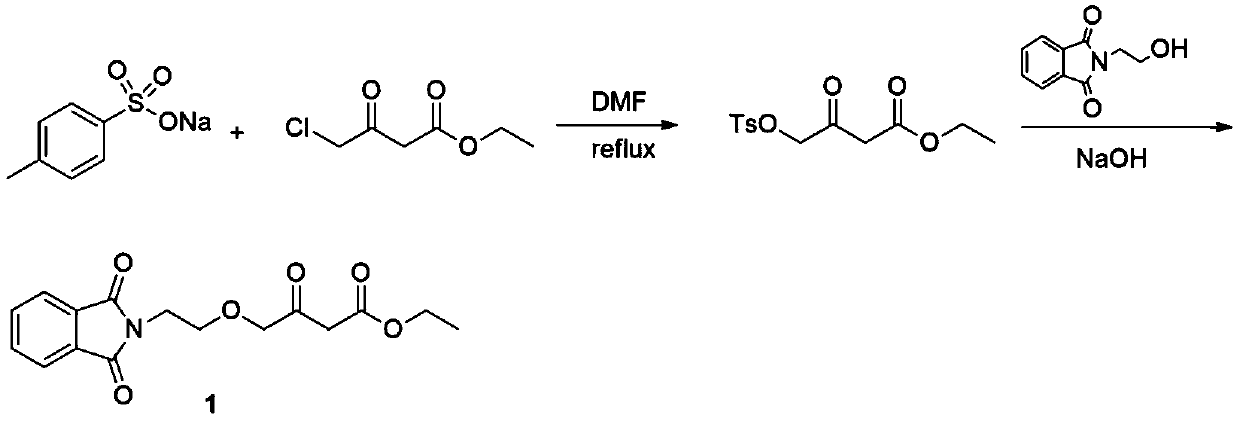
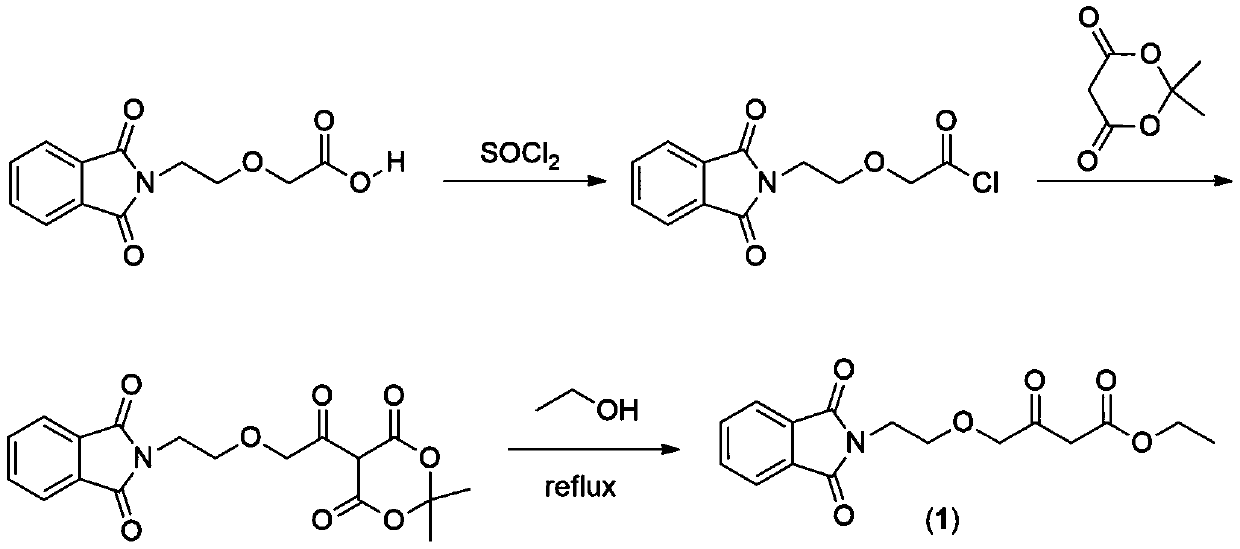
Preparation method of amlodipine key intermediate
A technology of amlodipine and intermediates, which is applied in the field of drug synthesis, can solve the problems of high price of McBurney's acid, increased equipment investment, raw materials and solvent costs, "three wastes" treatment costs, etc., and achieves easy large-scale industrial production and low raw material prices. Inexpensive and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of embodiment 1,2-(2-(1,3-dioxoisoindoline-2-yl) ethoxy) acetaldehyde
[0033]
[0034] Add 39.7g of oxalyl chloride in dichloromethane solution (200mL) into a 500mL three-necked flask, cool to -60°C, slowly drop in 30.9g of DMSO in dichloromethane solution (50mL), keep stirring for 0.5 h, add dropwise 47.8g of 2-[2-(2-hydroxyethoxy)ethyl]isoindoline-1,3-dione in dichloromethane solution (100mL), and keep warm at -60°C 2h, then slowly drop 61g of triethylamine, keep warm for 0.5h, then naturally warm up to room temperature. Wash 3 times with 5% dilute sulfuric acid (the aqueous phases are combined to adjust to alkaline, dichloromethane extraction can recover triethylamine, the recovery rate> 98%), the organic phase is washed 2 times with saturated brine, and dried over anhydrous sodium sulfate , filtered, and the solvent was evaporated to dryness to obtain a pale yellow oil, which was directly used in the next reaction without further purification. 1 ...
Embodiment 2
[0041] Embodiment 2, the synthesis of (2-(1,3-dioxaisoindoline-2-yl)ethoxy)-3-oxabutanoic acid ethyl ester (1)
[0042]
[0043] Add 3.5g of stannous chloride in dichloromethane solution (20mL) into a 500mL three-necked flask, cool to 0°C, dissolve 20.9g of ethyl diazoacetate in dichloromethane solution (80mL), dropwise into the three-necked flask, keep warm and stir for 10min, add 42.4g of (2-phthalimideethoxy) acetaldehyde in dichloromethane solution (150mL) into the three-necked flask, naturally raise the temperature to 25°C and keep it warm until The reaction ended (TLC monitored the progress of the reaction). The reaction solution was washed successively with saturated brine and saturated sodium thiosulfate solution, dried over anhydrous sodium sulfate, filtered, 2 g of silica gel was added to the filtrate, stirred for 0.5 h, filtered, and the filtrate was evaporated to dryness to obtain a light brown oil. 1 H NMR (300MHz, CDCl 3 )δ7.88(d×d, J=6.0Hz, 3.0Hz, 2H), 7.75...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com