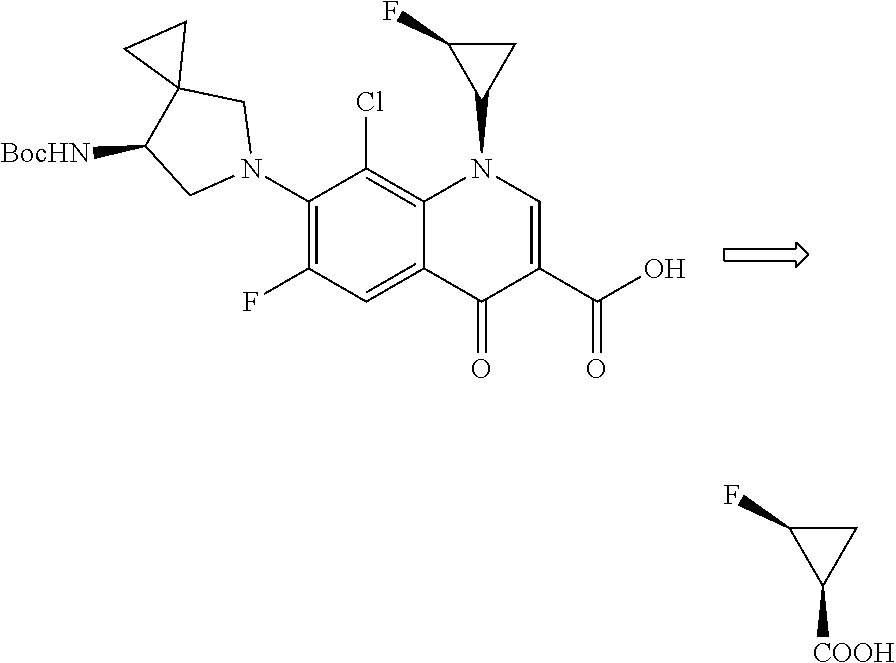
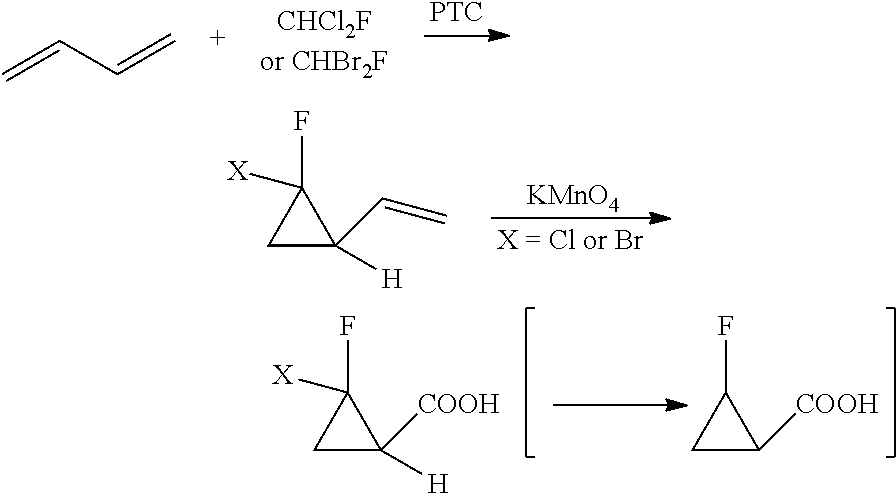
Method for synthesizing 2-fluorocyclopropane carboxylic acid
a technology of cyclopropane and carboxylic acid, which is applied in the preparation of carboxylic acid esters/lactones, organic chemistry, carboxylic compound preparations, etc., can solve the problems of high cost, high difficulty in synthesis of 2-fluorocyclopropane carboxylic acid, and high cost of sitafloxacin raw materials, etc., to achieve safe scale up, reduce production cost, and increase reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Example 1 for Step 1
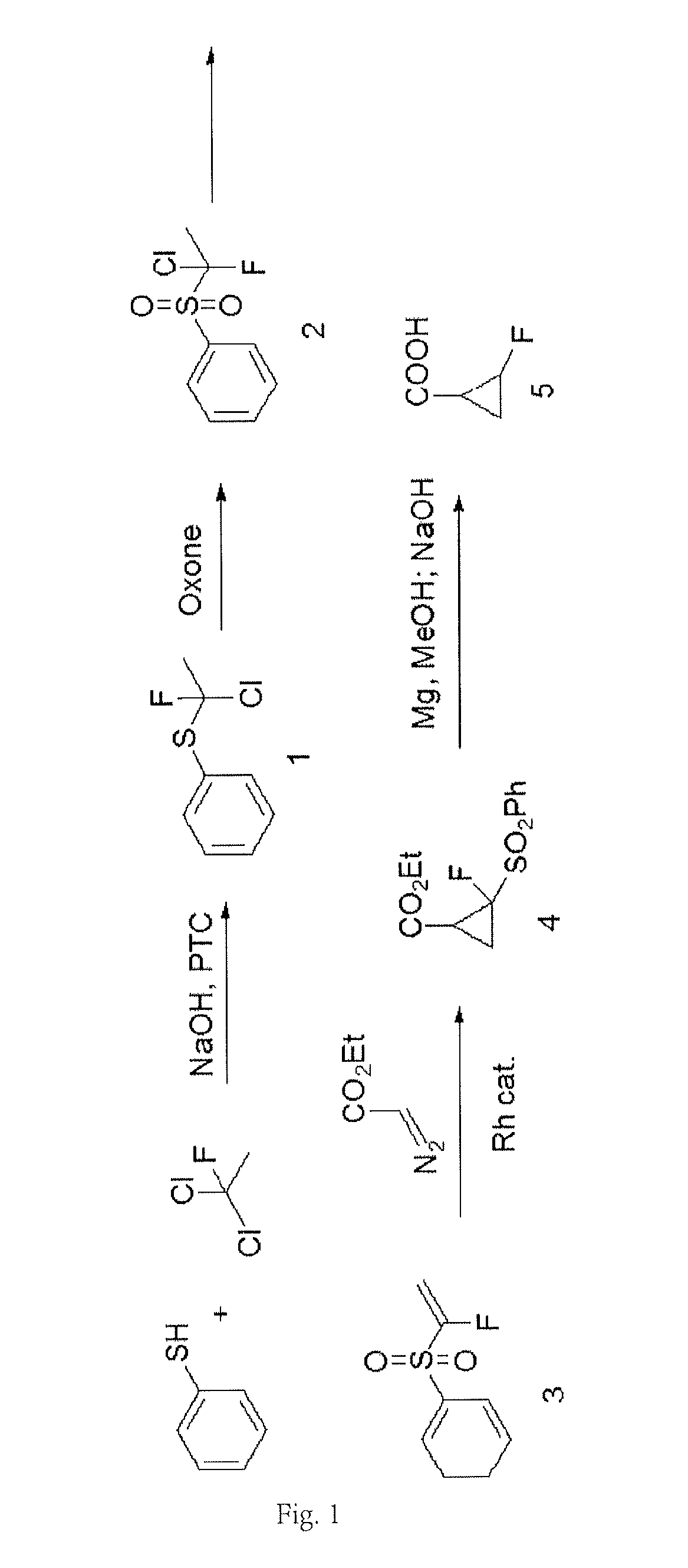
[0066]At room temperature, 10 g of thiophenol was added into 50 mL of methanol, and then 18 g of 40% NaOH solution was gradually added. 32 g of precooled 1, 1-dichloro-1-fluoroethane (Freon 141b) was added into the mixture, followed by stirring intensely overnight at 40-50° C. The reaction solution was cooled to room temperature, and 20 mL of concentrated hydrochloric acid was gradually added. The reaction solution was concentrated, so as to remove most of methanol. Then, it was extracted with ethyl acetate, washed with a saturated sodium carbonate solution, dried, and concentrated, so as to obtain 11 g of a crude product of a phenyl sulfide intermediate (Product 1 as shown in FIG. 1). The yield of the crude product was 59%.
Example 2 for Step 1
[0067]In an ice bath, 15 g of thiophenol, 20 g of 1,1-dichloro-1-fluoroethane, and 1.5 g of triethyl benzyl ammonium chloride were added into 100 mL of a toluene solution. After stirring, 80 mL of 50% sodium hydroxide solut...
example 1
for Step 3
[0070]11 g of potassium t-butoxide was dissolved in 100 mL of THF and cooled to 0° C. 15 g of the yellow oily product obtained in Step 2) was dissolved in 50 mL of THF and gradually added dropwise into the potassium t-butoxide solution. The temperature of the reaction solution was slowly increased to room temperature, followed by heating reflux overnight. After cooling, 200 mL of a saturated ammonium chloride solution was added and then concentrated to remove a part of THF. The reaction solution was extracted twice with ethyl acetate. The organic phase was washed with saturated sodium bicarbonate, dried, concentrated, followed by crystallization with n-hexane, so as to give 12 g of a light brown solid (Product 3 as shown in FIG. 1).
example 2
for Step 3
[0071]16 g of potassium hydroxide was dissolved in 12 mL of water and stirred for 0.5 h. Then, 12 g of methanol was gradually added. After stirring, 26 g of the yellow oily product obtained in Step 2) was added into the reaction solution. Then, the reaction solution was heated to 90° C. and hold for 3 hours, and then was cooled to room temperature. The reaction solution was extracted three times with methyl t-butyl ether. The organic phase was washed with saturated brine, dried, and concentrated, followed by crystallization with n-hexane, so as to give 18 g of a light brown solid (Product 3 as shown in FIG. 1).
Example for Step 4
[0072]17 g of the product obtained in Step 3) and 0.17 g of a rhodium triphenylacetate dimer catalyst were dissolved in 50 mL of methylene chloride, and then 12 g of ethyl diazoacetate in dichloromethane (40 mL) was gradually added dropwise. After stirring for 2 hours, the reaction solution was washed with diluted hydrochloric acid and washed with s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| mass ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com