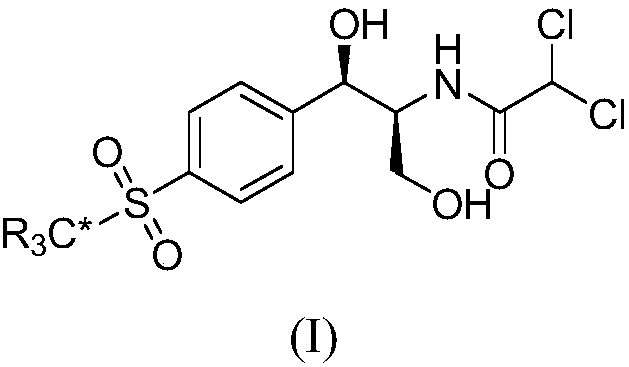
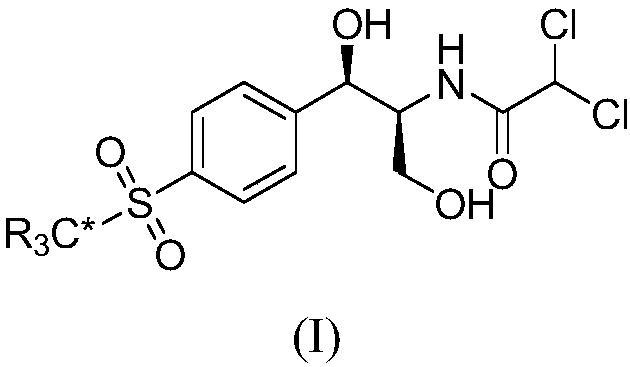
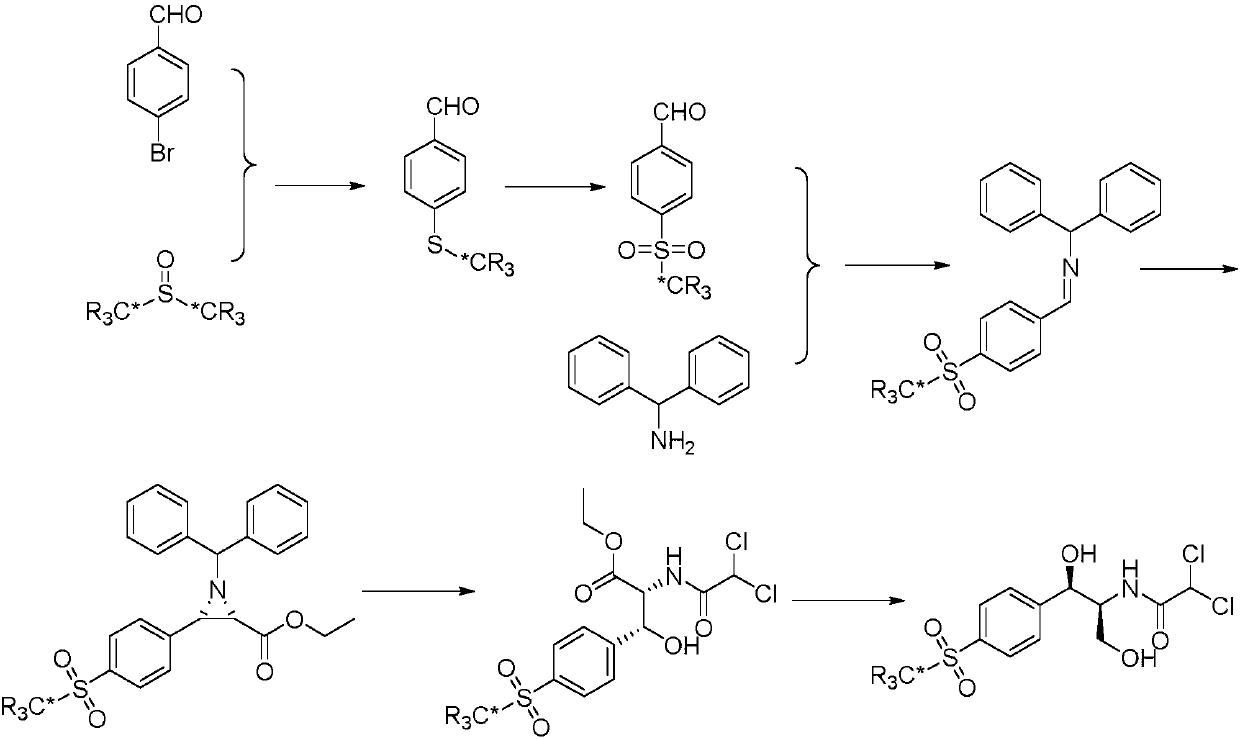
Synthetic method for stable isotope labeled thiamphenicol
A stable isotope and thiamphenicol technology, which is applied in organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of low total yield, low utilization rate of stable isotope-labeled intermediates, long routes, etc. problem, to achieve the effect of improving the total yield, simplifying the synthesis steps and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthetic method of deuterium-labeled 4-(methylmercapto) benzaldehyde
[0026]
[0027] In a sealed 25 mL glass tube, add p-bromobenzaldehyde (368 mg, 2 mmoL), cuprous iodide (76 mg, 0.4 mmoL), zinc acetate (364 mg, 4 mmoL), and dry deuterated dimethyl sulfoxide (6.5 mL) . Filled with nitrogen and reacted at 150°C for 12-36h. After the reaction was completed, it was cooled to room temperature, and the catalyst was removed by filtration. The filtrate was extracted with ethyl acetate (3×50 mL), the organic phases were combined, washed with water (3×50 mL), washed with saturated sodium chloride solution (3×50 mL), and dried over anhydrous sodium sulfate. After filtration, the solvent was removed to obtain a crude product, and then deuterium-labeled 4-(methylmercapto)benzaldehyde (263 mg, 85%) was obtained as a pure product by column chromatography. H NMR (CDCl 3 , 600M) δppm 9.92(s, 1H), 7.77(d, 2H), 7.32(d, 2H); MS ESI+156[M+1].
Embodiment 2
[0028] Embodiment 2: the synthetic method of deuterium-labeled 4-thiamphenicol benzaldehyde
[0029]
[0030] In a 50mL three-necked flask, add a mixed solution of water and n-butanol (10mL, volume ratio 1:1), add 30% hydrogen peroxide (2mL), stir well, add 4-(methylmercapto)benzaldehyde-methyl -D 3 (155mg, 1mmoL), stirred at 0-5°C for 2 hours. Filter, extract the filtrate with ethyl acetate (3×20mL), combine the organic phases, wash with saturated sodium bicarbonate solution (3×20mL), water (3×20mL), and saturated sodium chloride solution (3×20mL) , adding anhydrous sodium sulfate to dry. The desiccant was removed by filtration, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain deuterium-labeled 4-thiamphenicol benzaldehyde (172 mg, 92%). HNMR (CDCl 3 ,600M) δppm 9.88(s,1H), 7.81(d,2H), 7.36(d,2H); MS ESI+188[M+1].
Embodiment 3
[0031] Embodiment 3: The synthetic method of deuterium-labeled N-dianilino-1-(4-thiamphenicol phenyl) methylimine
[0032]
[0033] In a 100mL three-necked flask, add absolute ethanol (50mL), deuterated 4-thiamphenicol benzaldehyde (935mg, 5mmoL), and diphenylmethylamine (920mg, 5mmoL), react at 80°C for 2 hours, and cool to room temperature , the precipitated crystals were collected by filtration, washed with a small amount of cold ethanol, and dried under vacuum at 45° C. to obtain deuterium-labeled N-dianilino-1-(4-thiamphenylphenyl)methylimine (1.67 g, 95%). HNMR (CDCl 3 ,600M) δppm: 8.49(s,1H), 8.02(m,4H), 7.24~7.41(m,10H), 5.67(s,1H); MS ESI+353[M+1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com